Evaluating the Impact of Doxorubicin on rat testicular tissue and the protective role of Resveratrol and Thymoquinone
Abstract
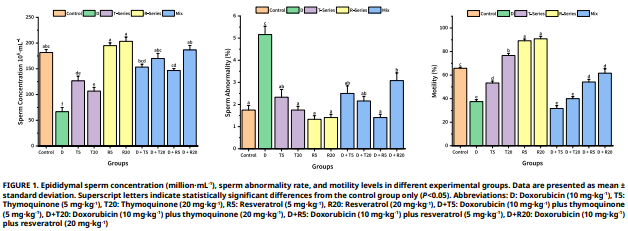
In this study, the harmful effects of doxorubicin on rat testicular tissue and the protective role of thymoquinone and resveratrol were investigated. The study employes 10 groups, each consisting of 8 male Wistar albino rats, for a total of 80 male rats. The groups were set up as follows: control (C), doxorubicin (D), resveratrol (R) (5 mg·kg-1, 20 mg·kg-1), thymoquinone (T) (5 mg·kg-1, 20 mg·kg-1), and combination groups (D + R 5 mg·kg-1, D + R 20 mg·kg-1, D + T 5 mg·kg-1, D + T 20 mg·kg-1). The C group received physiological saline by oral gavage every other day. Groups administered D at a dose of 15 mg·kg-1 intraperitoneally on day 10. Groups receiving T and R were given every other day for 21 days. At the end of the study period, the animals were euthanized by cervical dislocation under anesthesia. Testis and epididymis samples were collected with attention to sterility for spermatological, histopathological, and immunohistochemical analyses. The findings showed that sperm concentration decreased in all groups treated with D, motility and mitochondrial activity decreased, and acrosome and membrane integrity were impaired. Histopathological and immunohistochemical data also revealed significant decreases in germinal cell layer thickness, tubule diameter, Johnson’s testicular score, and relative testicular weight, while the B-cell lymphoma 2 (Bcl-2) ratio decreased and Bcl–2–associated X protein (Bax) expression increased (P<0.05). Additionally, the T 5 mg·kg-1 group exhibited an adverse effecton sperm density, motility, membrane integrity, and mitochondrial activity. The T 20 mg·kg-1, R 5 mg·kg-1, and R 20 mg·kg-1 groups, on the other hand, yielded more positive results than the control group for many parameters. When considering the combined groups, the D + R 5 mg·kg-1 and especially the D + R 20 mg·kg-1 groups successfully prevented the toxicity caused by the D group in terms of both spermatological and histopathological and immunohistochemical parameters. In conclusion, R was found to have a stronger protection against D-induced testicular toxicity compared to thymoquinone.
Downloads
References
Atıcı E. Tıp tarihinde kanser ve lösemi [Cancer and leukemia in the history of medicine]. Turk Onkol. Derg. [Internet]. 2007 [cited Aug 11, 2025]; 22(4):197–204. Turkish. Available in: https://goo.su/1DUdt
Sinha SJ, Kumar B, Prasad CP, Chauhan SS, Kumar M. Emerging research and future directions on doxorubicin: A snapshot. Asian Pac. J. Cancer Prev. [Internet]. 2025; 26(1):5–15. doi: https://doi.org/qn52 DOI: https://doi.org/10.31557/APJCP.2025.26.1.5
Sritharan S, Sivalingam N. A comprehensive review on time- tested anticancer drug doxorubicin. Life Sci. [Internet]. 2021; 278:119527. doi: https://doi.org/gmnsx7 DOI: https://doi.org/10.1016/j.lfs.2021.119527
Shinoda K, Mitsumori K, Yasuhara K, Uneyama C, Onodera H, Hirose M, Uehara M. Doxorubicin induces male germ cell apoptosis in rats. Arch. Toxicol. [Internet]. 1999; 73(4– 5):274–281. doi: https://doi.org/c7485x DOI: https://doi.org/10.1007/s002040050617
Brilhante O, Stumpp T, Miraglia SM. Long-term testicular toxicity caused by doxorubicin treatment during pre-pubertal phase. Int. J. Med. Med. Sci. [Internet]. 2011 [cited Aug 11, 2025]; 3(2):52–60. Available in: https://goo.su/4y64f
Hou M, Chrysis D, Nurmio M, Parvinen M, Eksborg S, Söder O, Jahnukainen K. Doxorubicin induces apoptosis in germ line stem cells in the immature rat testis and amifostine cannot protect against this cytotoxicity. Cancer. Res. [Internet]. 2005; 65(21):9999–10005. doi: https://doi.org/ddtgmb DOI: https://doi.org/10.1158/0008-5472.CAN-05-2004
Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Bauer JA. Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin. Pharmacol. Toxicol. [Internet]. 2005; 96(1):80–87. doi: https://doi.org/dnmcx4 DOI: https://doi.org/10.1111/j.1742-7843.2005.pto960112.x
Malik S, Singh A, Negi P, Kapoor VK. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today [Internet]. 2021; 26(11):2716–2725. doi: https://doi.org/gprscg DOI: https://doi.org/10.1016/j.drudis.2021.07.013
Aslani MR, Saadat S, Boskabady MH. Comprehensive and updated review on anti-oxidant effects of Nigella sativa and its constituent, thymoquinone, in various disorders. Iran. J. Basic Med. Sci. [Internet]. 2024; 27(8):923–951. doi: https://doi.org/qn54
Khan A. Antioxidant and anti-inflammatory action of thymoquinone. In: Younus H, editor. Molecular and therapeutic actions of thymoquinone. Singapore: Springer. [Internet]. 2018; 41–56. doi: https://doi.org/qn55 DOI: https://doi.org/10.1007/978-981-10-8800-1_4
Zhang LX, Li CX, Kakar MU, Khan MS, Wu PF, Amir RM, Dai DF, Naveed M, Li QY, Saeed M, Shen JQ, Rajput SA, Li JH. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. [Internet]. 2021; 143:112164. doi: https://doi.org/gq78cr DOI: https://doi.org/10.1016/j.biopha.2021.112164
Zini R, Morin C, Bertelli A, Bertelli A, Tillement J. Effects of resveratrol on the rat brain respiratory chain. Drugs. Exp. and Clin. Res. [Internet]. 1999 [cited Apr 01, 2025]; 25(2–3):87–97. Available in: https://goo.su/BJEPDy5
Okuizumi R, Harata R, Okamoto M, Sato S, Sugawara K, Aida Y, Nakamura A, Fujisawa A, Yamamoto Y, Kashiba M. Resveratrol is converted to the ring portion of coenzyme Q10 and raises intracellular coenzyme Q10 levels in HepG2 cell. J. Clin. Biochem. Nutr. [Internet]. 2024; 75(2):118–124. doi: https://doi.org/qn57 DOI: https://doi.org/10.3164/jcbn.24-70
Yan M, Zhao Y, Feng S, Zheng J, Diao M, Zhang T. Hydroxyl group-induced enhancement of antioxidant activity of resveratrol over pterostilbene by binding to lactoferrin. Food Chem. [Internet]. 2024; 441:138356. doi: https://doi.org/qn58 DOI: https://doi.org/10.1016/j.foodchem.2024.138356
Türedi S, Yuluğ E, Alver A, Kutlu Ö, Kahraman C. Effects of resveratrol on doxorubicin induced testicular damage in rats. Exp. Toxicol. Pathol. [Internet]. 2015; 67(3):229–235. doi: https://doi.org/qn59 DOI: https://doi.org/10.1016/j.etp.2014.12.002
Evans G, Maxwell WMC. Salamon’s artificial insemination of Sheep and Goats. London (UK): Butterworths-Heinemann; 1987
Türk G, Sönmez M, Çeribaşı AO, Yüce A, Ateşşahin A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil. Steril. [Internet]. 2008; 89(5):1474–1481. doi: https://doi.org/cxxqrg DOI: https://doi.org/10.1016/j.fertnstert.2007.04.059
Garner DL, Johnson LA. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. [Internet]. 1995; 53(2):276–284. doi: https://doi.org/d23cqf DOI: https://doi.org/10.1095/biolreprod53.2.276
Öztürk AE, Bodu M, Bucak MN, Ağır V, Özcan A, Keskin N, İli P, Topraggaleh TR, Sidal H, Başpınar N, Dursun S. The synergistic effect of trehalose and low concentrations of cryoprotectants can improve post-thaw ram sperm parameters. Cryobiology. [Internet]. 2020; 95:157–163. doi: https://doi.org/qn6b DOI: https://doi.org/10.1016/j.cryobiol.2020.03.008
Johnsen SG. Testicular biopsy score count – a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. [Internet]. 1970; 1(1):2–25. doi: https://doi.org/bvxfrs DOI: https://doi.org/10.1159/000178170
Shokoohi M, Shoorei H, Soltani M, Abtahi-Eivari SH, Salimnejad R, Moghimian M. Protective effects of the hydroalcoholic extract of Fumaria parviflora on testicular injury induced by torsion/detorsion in adult rats. Andrologia. [Internet]. 2018; 50(7):e13047. doi: https://doi.org/gh5vns DOI: https://doi.org/10.1111/and.13047
Hatipoğlu D, Ateş MB, Bodu M. Protective effects of Nigella sativa oil against bisphenol A-induced testicular toxicity in rats. Med. Weter. [Internet]. 2023; 79(1):28–35. doi: https://doi.org/qn6c
Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am. J. Pathol. [Internet]. 1996 [cited Jul 22, 2025]; 148(5):1567–1576. PMID: 8623925. Avilable in: https://goo.su/JHaUKXw
Özer H, Yenicesu G, Arici S, Cetin M, Tuncer E, Cetin A. Immunohistochemistry with apoptotic-antiapoptotic proteins (p53, p21, bax, bcl-2), c-kit, telomerase, and metallothionein as a diagnostic aid in benign, borderline, and malignant serous and mucinous ovarian tumors. Diagn. Pathol. [Internet]. 2012; 7(1):124. doi: https://doi.org/qn6d DOI: https://doi.org/10.1186/1746-1596-7-124
Levi M, Tzabari M, Savion N, Stemmer SM, Shalgi R, Ben-Aharon I. Dexrazoxane exacerbates doxorubicin-induced testicular toxicity. Reproduction [Internet]. 2015; 150(4):357–366. doi: https://doi.org/f7sgwn DOI: https://doi.org/10.1530/REP-15-0129
Shafiei-Roudbari SK, Malekinejad H, Janbaz-Aciabar H, Razi M. Crosstalk between E2F1 and P53 transcription factors in doxorubicin-induced DNA damage: evidence for preventive/ protective effects of silymarin. J. Pharm. Pharmacol. [Internet]. 2017; 69(9):1116–1124. doi: https://doi.org/qn6f DOI: https://doi.org/10.1111/jphp.12745
El-Maddawy ZK, Abd El Naby WSH. Protective effects of zinc oxide nanoparticles against doxorubicin-induced testicular toxicity and DNA damage in male rats. Toxicol. Res. [Internet]. 2019; 8(5):654–662. doi: https://doi.org/gppz3j DOI: https://doi.org/10.1039/c9tx00052f
Reddy KP, Madhu P, Reddy PS. Protective effects of resveratrol against cisplatin-induced testicular and epididymal toxicity in rats. Food Chem. Toxicol. [Internet]. 2016; 91:65–72. doi: https://doi.org/f8ng83 DOI: https://doi.org/10.1016/j.fct.2016.02.017
Archana D, Supriya C, Girish BP, Kishori B, Sreenivasula- Reddy P. Alleviative effect of resveratrol on polyvinyl chloride- induced reproductive toxicity in male Wistar rats. Food Chem. Toxicol. [Internet]. 2018; 116(B):173–181. doi: https://doi.org/qn6g DOI: https://doi.org/10.1016/j.fct.2018.04.026
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. [Internet]. 2006; 127(6):1109–1122. doi: https://doi.org/d5fr3k DOI: https://doi.org/10.1016/j.cell.2006.11.013
Zhu Z, Li R, Fan X, Lv Y, Zheng Y, Masudul-Hoque SA, Zeng W. Resveratrol improves boar sperm quality via 5′AMP-activated protein kinase activation during cryopreservation. Oxid. Med. Cell. Longev. [Internet]. 2019; 2019:5921503. doi: https://doi.org/qn6k DOI: https://doi.org/10.1155/2019/5921503
Simas JN, Mendes TB, Fischer LW, Vendramini V, Miraglia SM. Resveratrol improves sperm DNA quality and reproductive capacity in type 1 diabetes. Andrology [Internet]. 2021; 9(1):384–399. doi: https://doi.org/gpmqzb DOI: https://doi.org/10.1111/andr.12891
Atta MS, Almadaly EA, El-Far AH, Saleh RM, Assar DH, Al Jaouni SK, Mousa SA. Thymoquinone defeats diabetes- induced testicular damage in rats targeting antioxidant, inflammatory and aromatase expression. Int. J. Mol. Sci. [Internet]. 2017; 18(5):919. doi: https://doi.org/qn6m DOI: https://doi.org/10.3390/ijms18050919
Hussein SA, Khalaf-Allah SS, Tag El-Din HA, Amin A, Khallaf RM. Potential role of thymoquinone in imidaclopride-induced testicular toxicity in male albino rats. Benha Vet. Med. J. [Internet]. 2018; 34(3):64–82. doi: https://doi.org/qn6n DOI: https://doi.org/10.21608/bvmj.2018.40917
Salahshoor MR, Haghjoo M, Roshankhah S, Makalani F, Jalili C. Effect of thymoquinone on reproductive parameter in morphine-treated male mice. Adv. Biomed. Res. [Internet]. 2018; 7(1):18. doi: https://doi.org/gc2md8 DOI: https://doi.org/10.4103/abr.abr_69_17
Jalili C, Abdolmaleki A, Faramarzi A, Salahshoor MR. Effects of thymoquinone and busulfan on reproductive parameters in male rats: an experimental study. J. Clin. Diagn. Res. [Internet]. 2020; 14(1):1–4. doi: https://doi.org/qn6p DOI: https://doi.org/10.7860/JCDR/2020/42917.13404
Kopeika J, Kopeika E, Zhang T, Rawson DM. Studies on the toxicity of dimethyl sulfoxide, ethylene glycol, methanol and glycerol to loach (Misgurnus fossilis) sperm and the effect on subsequent embryo development. Cryoletters [Internet]. 2003 [cited Jul 22, 2025]; 24(6):365–374. Avaliable in: https://goo.su/Ybn9kaw
Verheijen M, Lienhard M, Schrooders Y, Clayton O, Nudischer R, Boerno S, Timmermann B, Selevsek N, Schlapbach R, Gmuender H, Gotta S, Geraedts J, Herwig R, Kleinjans J, Caiment F. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. [Internet]. 2019; 9(1):4641. doi: https://doi.org/gh39jk DOI: https://doi.org/10.1038/s41598-019-40660-0
Ijaz MU, Yaqoob S, Hamza A, David M, Afsar T, Husain FM, Amor H, Razak S. Apigetrin ameliorates doxorubicin prompted testicular damage: biochemical, spermatological and histological based study. Sci. Rep. 2024; 14(1):9049. doi: https://doi.org/qn6q DOI: https://doi.org/10.1038/s41598-024-59392-x
Radeva L, Yoncheva K. Doxorubicin Toxicity and Recent Approaches to Alleviating Its Adverse Effects with Focus on Oxidative Stress. Molecules [Internet] 2025; 30(15):3311. doi: https://doi.org/qn6r DOI: https://doi.org/10.3390/molecules30153311
Hassan E, El-Neweshy M, Hassan M, Noreldin A. Thymoquinone attenuates testicular and spermotoxicity following subchronic lead exposure in male rats: Possible mechanisms are involved. Life Sci. [Internet]. 2019; 230:132–140. doi: https://doi.org/qn6s DOI: https://doi.org/10.1016/j.lfs.2019.05.067
Mohammadi Z, Alaee S, Namavar MR, Khodabandeh Z, Ahmadi N, Rashidipour N, Karami-Mohajeri S. The antioxidant properties of resveratrol on sperm parameters, testicular tissue, antioxidant capacity, and lipid peroxidation in isoflurane- induced toxicity in mice. Hum. Exp. Toxicol. [Internet]. 2023; 42:09603271231215036. doi: https://doi.org/qn6t DOI: https://doi.org/10.1177/09603271231215036
Kang JK, Lee YJ, No K, Jung EY, Sung JH, Kim HJ, Nam SY. Ginseng intestinal metabolite-I (GIM-I) reduces doxorubicin toxicity in the mouse testis. Reprod. Toxicol. [Internet]. 2002; 16(3):291–298. doi: https://doi.org/b2trsd DOI: https://doi.org/10.1016/S0890-6238(02)00021-7
Das J, Ghosh J, Manna P, Sil PC. Taurine protects rat testes against doxorubicin-induced oxidative stress as well as p53, Fas and caspase 12-mediated apoptosis. Amino Acids. [Internet]. 2012; 42(5):1839–1855. doi: https://doi.org/bkc9f4 DOI: https://doi.org/10.1007/s00726-011-0904-4
Mendes TB, Paccola CC, de Oliveira Neves FM, Simas JN, da Costa Vaz A, Cabral REL, Vendramini V, Miraglia SM. Resveratrol improves reproductive parameters of adult rats varicocelized in peripuberty. Reproduction [Internet]. 2016; 152(1):23–35. doi: https://doi.org/f8t4qr DOI: https://doi.org/10.1530/REP-16-0025
Mabrouk A, Ben Cheikh H. Thymoquinone supplementation ameliorates lead-induced testis function impairment in adult rats. Toxicol. Ind. Health. [Internet]. 2016; 32(6):1114–1121. https://doi.org/g7t3hk DOI: https://doi.org/10.1177/0748233714548474
Sheikhbahaei F, Khazaei M, Rabzia A, Mansouri K, Ghanbari A. Protective effects of thymoquinone against methotrexate- induced germ cell apoptosis in male mice. Int. J. Fertil. Steril. [Internet]. 2016; 9(4):541–547. doi: https://doi.org/qn6v
Khames A, Khalaf MM, Gad AM, Abd El-Raouf OM, Kandeil MA. Nicorandil combats doxorubicin-induced nephrotoxicity via amendment of TLR4/P38 MAPK/NFκB signaling pathway. Chem. Biol. Interact. [Internet]. 2019; 311:108777. doi: https://doi.org/qn6w DOI: https://doi.org/10.1016/j.cbi.2019.108777