Examen histopatológico de la curación de defectos óseos en ayuno intermitente: un estudio experimental
Resumen
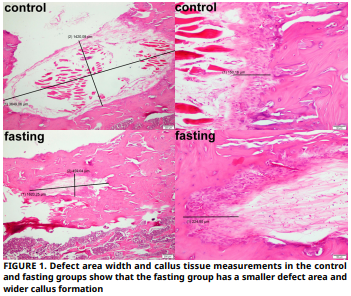
El objetivo de este estudio es examinar histopatológicamente el efecto del ayuno intermitente, que ha sido seguido con interés por los investigadores recientemente, en la curación de defectos óseos creados en tibias de ratas. Dieciséis ratas Sprague–Dawley se incluyeron en este estudio experimental. Para este propósito, las ratas se dividieron en dos grupos: un grupo de control del defecto (n = 8) y un grupo de defecto + ayuno (n = 8). En los grupos de defecto, se creó un defecto de 4 mm de diámetro y profundidad en el hueso corticoesponjoso de la tibia metafisaria. El ayuno intermitente se aplicó a los grupos de ayuno tres días a la semana durante ocho semanas. Todos los animales fueron sacrificados al final del proceso, y las tibias fueron descalcificadas y examinadas histopatológicamente, con nueva formación ósea y se evaluó el callo. Los datos se analizaron estadísticamente. La prueba t de Student se utilizó para el análisis estadístico. El tamaño medio del defecto longitudinal en el grupo control fue de 1675,43, mientras que el del grupo en ayunas fue de 1594,29. El tamaño medio del defecto vertical en el grupo control fue de 576,86, mientras que el del grupo en ayunas fue de 528. El tamaño medio del callo en el grupo control fue de 145, mientras que el del grupo en ayunas fue de 154,14. Tanto la formación ósea como los valores de callo fueron numéricamente más altos en el grupo de ayuno en comparación con el grupo control. Sin embargo, estas diferencias no fueron estadísticamente significativas (P>0,05). Dados los limitados resultados de este estudio, si bien el ayuno intermitente podría tener un posible efecto biológico en la consolidación ósea, no se encontraron diferencias estadísticamente significativas.
Descargas
Citas
Lelo I, Calabrese G, De Luca G, Conoci S. Recent advances in hydroxyapatite–based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. [Internet]. 2022; 23(17):9721. doi: https://doi.org/g8rzn6 DOI: https://doi.org/10.3390/ijms23179721
Omi M, Mishina Y. Roles of osteoclasts in alveolar bone remodeling. Genesis [Internet]. 2022; 60(8–9):e23490. doi: https://doi.org/gtz76m DOI: https://doi.org/10.1002/dvg.23490
Sun J, Xie W, Wu Y, Li Z, Li Y. Accelerated bone healing via electrical stimulation. Adv. Sci. [Internet]. 2025; 12(24):e2404190 doi: https://doi.org/g8694w DOI: https://doi.org/10.1002/advs.202404190
Fan S, Sun X, Su C, Xue Y, Song X, Deng R. Macrophages– bone marrow mesenchymal stem cells crosstalk in bone healing. Front. Cell Dev. Biol. [Internet]. 2023; 11:1193765. doi: https://doi.org/gtg2ph DOI: https://doi.org/10.3389/fcell.2023.1193765
Maruyama M, Rhee C, Utsunomiya T, Zhang N, Ueno M, Yao Z, Goodman SB. Modulation of the inflammatory response and bone healing. Front. Endocrinol. [Internet]. 2020; 11:386. doi: https://doi.org/gjtsz6 DOI: https://doi.org/10.3389/fendo.2020.00386
Moriarty TF, Metsemakers WJ, Morgenstern M, Hofstee MI, Diaz AV, Cassat JE, Wildemann B, Depypere M, Schwarz EM, Richards RG. Fracture–related infection. Nat. Rev. Dis. Primers. [Internet]. 2022; 8(1):67. doi: https://doi.org/mq9t DOI: https://doi.org/10.1038/s41572-022-00396-0
Wang YH, Zhao CZ, Wang RY, Du QX, Liu JY, Pan J. The crosstalk between macrophages and bone marrow mesenchymal stem cells in bone healing. Stem Cell Res. Ther. [Internet]. 2022; 13(1):511. doi: https://doi.org/qmxz DOI: https://doi.org/10.1186/s13287-022-03211-5
Zhao Q, Liu X, Yu C, Xiao Y. Macrophages and bone marrow– derived mesenchymal stem cells work in concert to promote fracture healing: A brief review. DNA Cell Biol. [Internet]. 2022; 41(3):276–284. doi: https://doi.org/grs8x6 DOI: https://doi.org/10.1089/dna.2021.0869
Khotib J, Gani MA, Budiatin AS, Lestari MLAD, Rahadiansyah E, Ardianto C. Signaling pathway and transcriptional regulation in osteoblasts during bone healing: Direct involvement of hydroxyapatite as a biomaterial. Pharmaceuticals [Internet]. 2021; 14(7):615. doi: https://doi.org/gp2cwb DOI: https://doi.org/10.3390/ph14070615
Molitoris KH, Huang M, Baht GS. Osteoimmunology of fracture healing. Curr. Osteoporos. Rep. [Internet]. 2024; 22(3):330–339. doi: https://doi.org/qmx2 DOI: https://doi.org/10.1007/s11914-024-00869-z
Shu LZ, Zhang XL, Ding YD, Lin H. From inflammation to bone formation: the intricate role of neutrophils in skeletal muscle injury and traumatic heterotopic ossification. Exp. Mol. Med. [Internet]. 2024; 56(7):1523–1530. doi: https://doi.org/g8xfdh DOI: https://doi.org/10.1038/s12276-024-01270-7
Camarena A, Kang L, Mirando AJ, Augustine E, McMillian NS, Stinson NC, Agarwal SM, Becker ML, Hilton MJ, Fernandez– Moure JS. Platelet–rich plasma enhances rib fracture strength and callus formation in vivo. J. Trauma Acute Care Surg. [Internet]. 2024; 97(6):884–890. doi: https://doi.org/qmx3 DOI: https://doi.org/10.1097/TA.0000000000004441
Trompet D, Melis S, Chagin AS, Maes C. Skeletal stem and progenitor cells in bone development and repair. J. Bone Miner. Res. [Internet]. 2024; 39(6):633–654. doi: https://doi.org/gt2h8d DOI: https://doi.org/10.1093/jbmr/zjae069
Hente RW, Perren SM. Tissue deformation controlling fracture healing. J. Biomech. [Internet]. 2021; 125:110576. doi: https://doi.org/qmx4 DOI: https://doi.org/10.1016/j.jbiomech.2021.110576
Kondi S, Gowda SR. Principles of bone healing. Surgery [Internet]. 2023; 41(10):625–631. doi: https://doi.org/qmx5 DOI: https://doi.org/10.1016/j.mpsur.2023.08.002
Duregon E, Pomatto–Watson LCDD, Bernier M, Price NL, de Cabo R. Intermittent fasting: from calories to time restriction. Geroscience [Internet]. 2021; 43(3):1083–1092. doi: https://doi.org/gm5crm DOI: https://doi.org/10.1007/s11357-021-00335-z
Hwangbo DS, Lee HY, Abozaid LS, Min KJ. Mechanisms of lifespan regulation by calorie restriction and intermittent fasting in model organisms. Nutrients [Internet]. 2020; 12(4):1194. doi: https://doi.org/g6xkh6 DOI: https://doi.org/10.3390/nu12041194
Hu D, Xie Z, Ye Y, Bahijri S, Chen M. The beneficial effects of intermittent fasting: an update on mechanism, and the role of circadian rhythm and gut microbiota. Hepatobiliary Surg. Nutr. [Internet]. 2020; 9(5):597–602. doi: https://doi.org/qmx6 DOI: https://doi.org/10.21037/hbsn-20-317
Song DK, Kim YW. Beneficial effects of intermittent fasting: a narrative review. J. Yeungnam Med. Sci. [Internet]. 2023; 40(1):4–11. doi: https://doi.org/qmx7 DOI: https://doi.org/10.12701/jyms.2022.00010
Elsworth RL, Monge A, Perry R, Hinton EC, Flynn AN, Whitmarsh A, Hamilton–Shield JP, Lawrence NS, Brunstrom JM. the effect of intermittent fasting on appetite: A systematic review and meta–analysis. Nutrients [Internet]. 2023; 15(11):2604. doi: https://doi.org/qmx8 DOI: https://doi.org/10.3390/nu15112604
Clayton DJ, Varley I, Papageorgiou M. Intermittent fasting and bone health: a bone of contention? Br. J. Nutr. [Internet]. 2023; 130(9):1487–1499. doi: https://doi.org/qmx9 DOI: https://doi.org/10.1017/S0007114523000545
Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat. Rev. Endocrinol. [Internet]. 2022; 18(5):309–321. doi: https://doi.org/gqrts3 DOI: https://doi.org/10.1038/s41574-022-00638-x
Serger E, Luengo–Gutierrez L, Chadwick JS, Kong G, Zhou L, Crawford G, Danzi MC, Myridakis A, Brandis A, Bello AT, Müller F, Sanchez–Vassopoulos A, De Virgiliis F, Liddell P, Dumas ME, Strid J, Mani S, Dodd D, Di Giovanni S. The gut metabolite indole–3 propionate promotes nerve regeneration and repair. Nature [Internet]. 2022; 607(7919):585–592. doi: https://doi.org/gqd3nr DOI: https://doi.org/10.1038/s41586-022-04884-x
Minciuna I, Gallage S, Heikenwalder M, Zelber–Sagi S, Dufour JF. Intermittent fasting–the future treatment in NASH patients? Hepatology [Internet]. 2023; 78(4):1290–1305. doi: https://doi.org/gs86nz DOI: https://doi.org/10.1097/HEP.0000000000000330
Zeb F, Wu X, Chen L, Fatima S, Haq IU, Chen A, Majeed F, Feng Q, Li M. Effect of time–restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. [Internet]. 2020; 123(11):1216–1226. doi: https://doi.org/qmzb DOI: https://doi.org/10.1017/S0007114519003428
Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Taub PR. Ten–Hour time–restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. [Internet]. 2020; 31(1):92–104.e5. doi: https://doi.org/gghdcg DOI: https://doi.org/10.1016/j.cmet.2019.11.004
Papageorgiou M, Biver E, Mareschal J, Phillips NE, Hemmer A, Biolley E, Schwab N, Manoogian ENC, Gonzalez Rodriguez E, Aeberli D, Hans D, Pot C, Panda S, Rodondi N, Ferrari SL, Collet TH. The effects of time–restricted eating and weight loss on bone metabolism and health: a 6–month randomized controlled trial. Obesity (Silver Spring). [Internet]. 2023; 31(1):85–95. doi: https://doi.org/qmzc DOI: https://doi.org/10.1002/oby.23577
McAllister MJ, Pigg BL, Renteria LI, Waldman HS. Time– restricted feeding improves markers of cardiometabolic health in physically active college–age men: a 4–week randomized pre–post pilot study. Nutr. Res. [Internet]. 2020; 75:32–43. doi: https://doi.org/gpnnf2 DOI: https://doi.org/10.1016/j.nutres.2019.12.001
Martens CR, Rossman MJ, Mazzo MR, Jankowski LR, Nagy EE, Denman BA, Richey JJ, Johnson SA, Ziemba BP, Wang Y, Peterson CM, Chonchol M, Seals DR. Short–term time– restricted feeding is safe and feasible in non–obese healthy midlife and older adults. Geroscience [Internet]. 2020; 42(2):667–686. doi: https://doi.org/qmzd DOI: https://doi.org/10.1007/s11357-020-00156-6
Veronese N, Reginster JY. The effects of calorie restriction, intermittent fasting and vegetarian diets on bone health. Aging Clin. Exp. Res. [Internet]. 2019; 31(6):753–758. doi: https://doi.org/gfzjjg DOI: https://doi.org/10.1007/s40520-019-01174-x
Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, Lu L, Yao Z, Goodman SB. Mesenchymal stem cell–macrophage crosstalk and bone healing. Biomaterials [Internet]. 2019; 196:80–89. doi: https://doi.org/gh4vp4 DOI: https://doi.org/10.1016/j.biomaterials.2017.12.025
Tanrisever M, Tekin B, Can UK, Istek O, Ozcan EC, Ozercan IH, Gelic T, Dundar S.The effect of local melatonin application on bone fracture healing in Rat tibias. Medicina [Internet]. 2025; 61(1):146. doi: https://doi.org/qmzf DOI: https://doi.org/10.3390/medicina61010146
Dalle–Carbonare L, Cominacini M, Trabetti E, Bombieri C, P es soa J , R omanel li MG, Valenti MT.The bone microenvironment: new insights into the role of stem cells and cell communication in bone regeneration. Stem Cell Res. Ther. [Internet]. 2025; 16(1):169. doi: https://doi.org/qmzg DOI: https://doi.org/10.1186/s13287-025-04288-4
Sheen JR, Mabrouk A, Garla VV. Fracture Healing Overview. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 (cited Oct 26, 2025). Available in: https://goo.su/H0c5t
Sequeira I. Aging: Restoring bone healing potential. Elife [Internet]. 2025; 14:105420. doi: https://doi.org/qmzh DOI: https://doi.org/10.7554/eLife.105420
Reeves J, Tournier P, Becquart P, Carton R, Tang Y, Vigilante A, Fang D, Habib SJ.Rejuvenating aged osteoprogenitors for bone repair. Elife [Internet]. 2024; 13:104068. doi: https://doi.org/qmzj DOI: https://doi.org/10.7554/eLife.104068.3
Liang W, Zhou C, Liu X, Xie Q, Xia L, Liu L, Bao W, Lin H, Xiong X, Zhang H, Zheng Z, Zhao J.Current status of nano–embedded growth factors and stem cells delivery to bone for targeted repair and regeneration. J. Orthop. Transl. [Internet]. 2025; 50:257–273. doi: https://doi.org/qmzk DOI: https://doi.org/10.1016/j.jot.2024.12.006
He T, Qin L, Chen S, Huo S, Li J, Zhang F, Yi W, Mei Y, Xiao G. Bone–derived factors mediate crosstalk between skeletal and extra–skeletal organs. Bone Res. [Internet]. 2025; 13(1):49. doi: https://doi.org/qmzm DOI: https://doi.org/10.1038/s41413-025-00424-1
Iyer SS. Epigenetic regulation of bone healing: implications for fracture repair and clinical treatment strategies. Yale J. Biol. Med. [Internet]. 2025; 98(2):159–170. doi: https://doi.org/qmzn DOI: https://doi.org/10.59249/HSYL8000
Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. Importance of systematic reviews and meta–analyses of animal studies: Challenges for animal–to–human translation. J. Am. Assoc. Lab. Anim. Sci. [Internet]. 2020; 59(5):469–477. doi: https://doi.org/gg637h DOI: https://doi.org/10.30802/AALAS-JAALAS-19-000139
Gileta AF, Fitzpatrick CJ, Chitre AS, St Pierre CL, Joyce EV, Maguire RJ, McLeod AM, Gonzales NM, Williams AE, Morrow JD, Robinson TE, Flagel SB, Palmer AA. Genetic characterization of outbred Sprague Dawley rats and utility for genome–wide association studies. PLoS Genet. [Internet]. 2022; 18(5):e1010234. doi: https://doi.org/g9stfs DOI: https://doi.org/10.1371/journal.pgen.1010234
Polat E, Bostan–Yoru HG, Kalay Y, Akca S. The importance of screening programs in laboratory animals and investigation of several important viral agents. J. Lab. Anim. Sci. Pract. [Internet]. 2024; 4(2):91–100. doi: https://doi.org/qmzp DOI: https://doi.org/10.62425/jlasp.1484805
Durmaz B, Gunes N, Koparal M, Gul M, Dundar S, Bingul MB. Investigation of the effects of quercetin and xenograft on the healing of bone defects: An experimental study. J. Oral Biol. Craniofac. Res. [Internet]. 2023; 13(1):22–27. doi: https://doi.org/pmp9 DOI: https://doi.org/10.1016/j.jobcr.2022.10.008