Sustitución de heno de alfalfa por Rumex pulcher L. y su efecto sobre la producción de gas in vitro y fermentación ruminal
Resumen
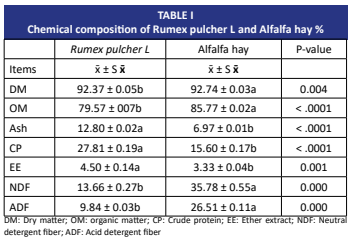
En este estudio, se determinaron los efectos del forraje de Rumex pulcher L. añadido al heno de alfalfa como fuente alternativa de forraje en rumiantes, en diferentes proporciones de mezcla (0, 25, 50 y 100 %), sobre la producción de gas y metano, las propiedades de digestibilidad in vitro y los parámetros de fermentación ruminal, mediante la técnica de producción de gas in vitro. Se conformaron los siguientes grupos experimentales: control (C: 100 % heno de alfalfa), forraje de Rumex pulcher L. 1 (75 % heno de alfalfa + 25 % forraje de Rumex pulcher L.), forraje de Rumex pulcher L. 2 (50 % heno de alfalfa + 50 % forraje de Rumex pulcher L.) y forraje de Rumex pulcher L. 3 (100 % forraje de Rumex pulcher L.). Cada grupo experimental se diseñó con 5 repeticiones. El forraje de Rumex pulcher L. presentó un contenido significativamente mayor (P < 0,001) de proteína cruda y menor de fibra detergente neutra y fibra detergente ácida que el heno de alfalfa. Si bien la producción de gas y metano no varió en ninguno de los tratamientos, el forraje de Rumex pulcher L. 3 mostró la mayor digestibilidad de la materia orgánica en comparación con los demás grupos. A medida que aumentaron las tasas de forraje de Rumex pulcher L., se observó un incremento en los parámetros de síntesis de proteína microbiana. Los parámetros ruminales, a excepción del ácido acético, presentaron diferencias entre los tratamientos. La forraje de Rumex pulcher L. puede incorporarse a las raciones de rumiantes para reducir los costos de alimentación, debido a sus propiedades superiores en comparación con el heno de alfalfa en cuanto a composición nutricional, digestibilidad in vitro y síntesis de proteína microbiana. Además, es importante realizar estudios adicionales sobre esta especie, que crece de forma silvestre, y explorarla como fuente alternativa de forraje.
Descargas
Citas
Rojas-Downing MM, Nejadhashemi AP, Harrigan T, Woznicki SA. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. [Internet]. 2017; 16:145-163. doi: https://doi.org/gfvdh6 DOI: https://doi.org/10.1016/j.crm.2017.02.001
Halmemies-Beauchet-Filleau A, Rinne M, Lamminen M, Mapato C, Ampapon T, Wanapat M, Vanhatalo A. Review: Alternative and novel feeds for ruminants: nutritive value, product quality and environmental aspects. Animal [Internet]. 2018; 12(2):295-309. doi: https://doi.org/gffvw6 DOI: https://doi.org/10.1017/S1751731118002252
Wanapat M, Cherdrhong A, Phesatcha K, Kang S. Dietary sources and their effects on animal production and environmental sustainability. Anim. Nutr. [Internet]. 2015; 1(3):96-103. doi: https://doi.org/gfzm9h DOI: https://doi.org/10.1016/j.aninu.2015.07.004
Kuruüzüm A, Demirezer Ö. Rumex türlerinin kullanılışı ve biyolojik aktiviteleri. FABAD J. Pharm. Sci. 1997 [cited 20 Jul 2025]; 22:21-26. Available in: https://goo.su/zWDbh7
Sabuncu M, Konak M, Şahan Y. Rumex acetosella L’nin biyoalınabilir antioksidan özelliklerinin belirlenmesi. Bursa Uludag Üniv. Ziraat Fak. Derg. [Internet]. 2019 [cited 20 Jul 2025]; 33(2):197-207. Available in: https://goo.su/0G5mz7
Güney M, Aydın R. Evelik otu’nun (Rumex acetosella) yem değeri ve in vitro gerçek sindirilebilirliğinin belirlenmesi. Yuzuncu Yil Üniv. Fen Bilimleri Enst. Derg. [Internet]. 2024; 29(2):780-786. doi: https://doi.org/qjgb DOI: https://doi.org/10.53433/yyufbed.1486178
Taşkın T. Çakırlı (Bursa-Orhangazi) yöresinde yenebilen bazı yabani bitkilerin antioksidan aktivitelerinin incelenmesi. [master thesis on Internet]. Türkiye: Marmara Üniversitesi, Sağlık BilimLeri Enstitüsü. 2011 [cited 20 Jul 2025]. 129 p. Available in: https://goo.su/UKGLOOg
Barros-Rodriguez M, Rovalino-Nunez V, Núñez-Torres O, Mera-Andrade R, Artieda-Rojas J, Vaca-Freire L, Curay- Quispe S, Ortiz-Tirado P, Solorio-Sanchez J, Iraola J. Composición química, cinética de degradación ruminal y producción de gas in vitro de arvenses con potencial forrajero. Livest. Res. Rural Dev. [Internet]. 2017 [cited 20 jul 2025]; 29(4):1-12. Available in: https://goo.su/qnqFI
Association of Official Analytical Chemistry (AOAC). Official Methods of Analysis. 18th ed. Washington, DC: AOAC International; 2010.
Ankom. Crude fiber analysis in feeds by filter bag technique. Macedon, NY: ANKOM Technology; 2008 [cited 20 Jul 2025]. https://goo.su/GU922l
Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. [Internet]. 1991; 74: 3583-3597.doi: https://doi.org/b6c78f DOI: https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Menke KH. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. [Internet]. 1988; 28:7-55. Available in: https://goo.su/6eMAIqN
Blümmel M, Steingass H, Becker K. The relationship between in vitro gas production, in vitro microbial biomass yield and N-15 incorporation and its implications for the prediction of voluntary feed intake of roughages. Br. J. Nutr. [Internet]. 1997; 77(6): 911-921. doi: https://doi.org/dq2dpc DOI: https://doi.org/10.1079/BJN19970089
Markham R. A steam distilation apparatus suitable for micro-kjeldahl analyses. J. Biochem. [Internet]. 1942; 36(10-12):790-791. doi: https://doi.org/qjgc DOI: https://doi.org/10.1042/bj0360790
Senyuz HH, Karsli MA. The substitution of corn silage with potato pulp silage at differing level in dairy cows on milk yield, composition and rumen volatile fatty acids. J. Fac. Vet. Med. Erciyes Univ. [Internet]. 2021; 18(1): 1-10. doi: https://doi.org/qjgd DOI: https://doi.org/10.32707/ercivet.872993
Castillos-Vargas JA, Costa de Araújo T, Mezzomo R. Extraction, identification, and quantification of volatile fatty acids (VFA) in rumen fluid samples using Reverse Phase High-Performance Liquid Chromatography with Diode Array Detector (RP HPLC-DAD). Res. Sq. [Internet]. 2020; 1:1-11. doi: https://doi.org/qjgf DOI: https://doi.org/10.21203/rs.3.pex-1121/v1
Statistical Analysis System (SAS). SAS/STAT Software: Hangen and Enhanced, Version 9.4, Cary, North Carolina, USA: SAS Institute Inc. 2014.
Weiss B. Feeding high ash forages. College of Food, Agricultural, and Environmental Sciences, Ohio State University Extension. Columbus, Ohio: Ohio State University. [Internet]. 2020 [cited 20 Jul 2025]. Available in: https://goo.su/cpAmPm
Kılıç Ü, Abdiwali MA. Alternatif kaba yem kaynağı olarak şarapçılık endüstrisi üzüm atıklarının in vitro gerçek sindirilebilirlikleri ve nispi yem değerlerinin belirlenmesi. Kafkas Üniv. Vet. Fak. Derg. [Internet]. 2016; 22(6):895- 901. doi: https://doi.org/qjgj
Suárez-Ramos G, Serrano-Cárdenas V, Pelz-Marín R, Balderas-Aguilar P. Atlas de malezas arvenses del estado de Querétaro. 1ª ed. México: Universidad Autónoma de Querétaro . 2004. 255 p.
Çelik H, Selçuk Z. Karambanın fiğ otu ve yonca otu ile farklı oranda karışımLarının in vitro gerçek sindirilebilirliğinin belirlenmesi. Van Vet. J. [Internet]. 2019; 30(3):145-149. doi: https://doi.org/qjj3 DOI: https://doi.org/10.36483/vanvetj.478518
Bilal Y, Bakır T, Selçuk B. Evelik otunun (Rumex acetosella) kuzu rasyonlarına ilavesinin sindirim derecesine ve fermantasyon parametrelerine etkisinin in vitro gaz üretim tekniği ile belirlenmesi. KSÜ Tarım ve Doğa Derg. (Internet). 2023; 26(4):911-918. doi: https://doi.org/qjj4 DOI: https://doi.org/10.18016/ksutarimdoga.vi.1195753
Ateş E. The mineral, amino acid and fiber contents and forage yield of pea (Pisum arvense L.), fiddleneck (Phacelie tanacetifolia Benth.) and their mixtures under dry land conditions in the Western Turkey. Romanian Agric. Res. [Internet]. 2012; 29(29):237-244. Available in: https://goo.su/P92N8sF
Martínez-Loperena R, Castelán-Ortega OA, Gonzáles- Ronquillo, M, Estrada-Flores JGE. Nutritive value, in vitro fermentation and secondary metabolites of weeds and maize straw used for feeding dairy cattle. Trop. Subtrop. Agroecosyst. [Internet]. 2011; 14(2):525-536. Available in: https://goo.su/d241
Tavendale MH, Lane GA, Schreurs NM, Fraser K, Meagher LP. The effects of condensed tannins from Dorycnium rectum on skatole and indole ruminal biogenesis for grazing sheep. Aust. J. Agric. Res. [Internet]. 2005; 56(12):1331-1337. doi: https://doi.org/d5zxz3 DOI: https://doi.org/10.1071/AR04232
Waghorn GC, Woodward SL. Ruminant contributions to methane and global warming-a New Zealand perspective. In: Bhatti JS, Lal R, Apps MJ, Price MA, editors. Climate Change and Managed Ecosystems. Boca Raton, FL: Taylor & Francis Group; 2006. p. 233-260. DOI: https://doi.org/10.1201/9781420037791.ch12
Özkan ÇÖ, Cengiz T, Yanık M, Evlice S. Selçuk B, Ceren B, Kamalak A. Ruminant hayvan beslemede kullanılan bazı kaba ve kesif yemLerin in vitro gaz üretiminin, metan üretiminin, sindirim derecesinin ve mikrobiyal protein üretiminin belirlenmesi. Black Sea J. Agric. [Internet]. 2020; 3(1): 56-60. Available in: https://goo.su/Egj9W0J
Van Soest PJ. Nutritional ecology of the ruminant. 2nd ed. Ithaca, NY, USA: Cornell University Press; 1994. DOI: https://doi.org/10.7591/9781501732355
Ece Z, Avcı M. Yonca kuru otu ve süt sığırı rasyonuna zeolit ve meşe palamudu ilavesinin in vitro organik madde sindirimi ve metan oluşumu üzerine etkisi. Harran Üniv. Vet. Fak. Derg. [Internet]. 2018; 7(1):67-73. doi: https://doi.org/qjkd DOI: https://doi.org/10.31196/huvfd.470790
Bach A, Calsamiglia S, Stern MD. Nitrogen metabolism in the rumen. J. Dairy Sci. [Internet]. 2005; 88:E9-E21. doi: https://doi.org/d2h7xj DOI: https://doi.org/10.3168/jds.S0022-0302(05)73133-7
Clark JH, Klusmeyer TH, Cameron MR. Microbial protein synthesis and flows of nitrogen fractions to the duodenum of dairy cows. J. Dairy Sci. [Internet]. 1992; 75(8):2304-2323. doi: https://doi.org/bg2tz6 DOI: https://doi.org/10.3168/jds.S0022-0302(92)77992-2
Bilal Y, Kamalak A. Kuzu rasyonlarına meşe palamudu ilavesinin sindirim derecesine, metabolik enerjisine ve metan üretimine etkisinin in vitro gaz üretim tekniği ile belirlenmesi. KSU J. Agric. Nat. [Internet]. 2022; 25(2):583-590. doi: https://doi.org/qjkz DOI: https://doi.org/10.18016/ksutarimdoga.vi.1116585
Blümmel M, Lebzien P. Predicting ruminal microbial efficiencies of dairy rations by in vitro techniques. Livest. Prod. Sci. [Internet]. 2001; 68(2-3):107-117. doi: https://doi.org/bvq7hk DOI: https://doi.org/10.1016/S0301-6226(00)00241-4
Vargas JE, López-Ferreras L, Andrés S, Mateos I, Horst EH, López S. Differential diet and pH effects on ruminal microbiota, fermentation pattern and fatty acid hydrogenation in RUSITEC continuous cultures. Fermentation. [Internet]. 2023; 9(4):320. doi: https://doi.org/qjk3 DOI: https://doi.org/10.3390/fermentation9040320
Putri EM, Zain M, Warly ML, Hermon H. Effects of rumen- degradable-to-undegradable protein ratio in ruminant diet on in vitro digestibility, rumen fermentation, and microbial protein synthesis. Vet. World. [Internet]. 2021; 14(3):640-648. doi: https://doi.org/qjk4 DOI: https://doi.org/10.14202/vetworld.2021.640-648
Uddin MD, Haque KZ, Jasimuddin KM, Hasan KMM. Dynamics of microbial protein synthesis in the rumen a review. Ann. Vet. Anim. Sci. [Internet]. 2015 [cited 22 May 2025]; 2(5):116-131. Available in: https://goo.su/gX8abB
Bahrami-Yekdangi M, Ghorbani GR, Khorvash M, Khan MA, Ghaffari MH. Reducing crude protein and rumen degradable protein with a constant concentration of rumen undegradable protein in the diet of dairy cows: Production performance, nutrient digestibility, nitrogen efficiency, and blood metabolites. J. Anim. Sci. [Internet]. 2016; 94(2):718-725. doi: https://doi.org/f8nfm6 DOI: https://doi.org/10.2527/jas.2015-9947
Yang C, Bing-Wen S, Qi-Yu D, Hai J, Shu-Qin Z, Yan T. Rumen fermentation and bacterial communities in weaned Chahaer lambs on diets with different protein levels. J. Integr. Agric. [Internet]. 2016; 15(7):1564-1574. doi: https://doi.org/f8wqqw DOI: https://doi.org/10.1016/S2095-3119(15)61217-5
Paula EM, Monteiro HF, Silva LG, Benedeti BDB, Daniel JLP, Shenkoru T, Broderick GA, Faciola AP. Effects of replacing soybean meal with canola meal differing in rumen-undegradable protein content on ruminal fermentation and gas production kinetics using 2 in vitro systems. J. Dairy Sci. [Internet]. 2017; 100(7):5281-5292. doi: https://doi.org/gbkbkn DOI: https://doi.org/10.3168/jds.2016-12301