Comparative phytochemical composition and bioactivities of local variety of Prunus domestica L. from Northeastern Türkiye
Abstract
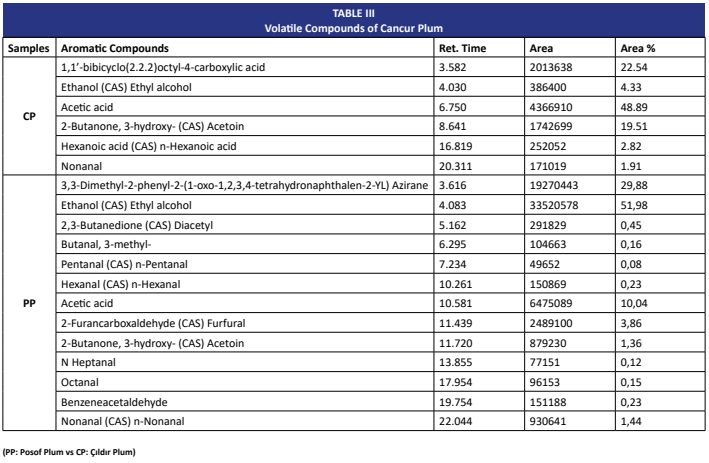
The Cancur plum (Prunus domestica L.), a local variety grown in Northeastern Türkiye, represents a valuable source of bioactive compounds with potential functional food applications. This study aimed to comprehensively evaluate the phytochemical composition and functional properties of Cancur plum fruits collected from two regions with contrasting climatic conditions: Posof (microclimate, 1260 m) and Çıldır (continental climate, 1585 m). Antioxidant potential was assessed using multiple approaches, including TAC, DPPH, glutathione, SOD, and catalase assays, while the quantities of total phenolics, flavonoids, and anthocyanins were also determined. The characterization of individual phenolic and flavonoid molecules was performed via LC-MS/MS, volatile constituents were profiled through GC- MS, and free sugars were quantified using HPLC. Comparative evaluation showed that the Çıldır vinegars exhibited greater DPPH radical scavenging capacity and elevated catalase activity, whereas the Posof vinegars were richer in total phenolics and glutathione. Despite these differences, both groups displayed a similar overall antioxidant capacity, though mediated by distinct biochemical mechanisms. LC-MS/MS profiling highlighted shikimic, chlorogenic, and p-coumaric acids as the predominant phenolics, with rutin and hesperidin occurring in higher amounts in the Çıldır sample. In terms of aroma-active compounds, acetic acid and acetoin dominated in the Çıldır vinegars, while ethanol and furfural were more pronounced in the Posof samples.Glucose and fructose were identified as the primary sugars, with minor sucrose detected only in Çıldır fruits. Microclimatic differences strongly shape the biochemical and functional profiles of Cancur plum, highlighting its value as a source of antioxidants, flavor compounds, and functional food ingredients.
Downloads
References
Oz AT, Kafkas E. Phytochemicals in Fruits and Vegetables. In: Waisundara V, Shiomi N, (editors). Superfood Funct. Food Process. Util. InTech. [Internet]. 2017; 8:175-184. doi: https://doi.org/qg56 DOI: https://doi.org/10.5772/66987
Hashim MS, Lincy S, Remya V, Teena M, Anila L. Effect of polyphenolic compounds from Coriandrum sativum on H2O2-induced oxidative stress in human lymphocytes. Food Chem. [Internet]. 2005; 92(4):653-660. doi: https://doi.org/bpk94r DOI: https://doi.org/10.1016/j.foodchem.2004.08.027
Lima GPP, Vianello F, Corrêa CR, da Silva Campos RA, Borguini MG. Polyphenols in Fruits and Vegetables and Its Effect on Human Health. Food Nutr. Sci. [Internet]. 2014; 5(11):1065-1082. doi: https://doi.org/qg57 DOI: https://doi.org/10.4236/fns.2014.511117
Kristl J, Slekovec M, Tojnko S, Unuk T. Extractable antioxidants and non-extractable phenolics in the total antioxidant activity of selected plum cultivars (Prunus domestica L.): Evolution during on-tree ripening. Food Chem. [Internet]. 2011; 125(1):29-34. doi: https://doi.org/dcs68v DOI: https://doi.org/10.1016/j.foodchem.2010.08.027
Rop O, Jurikova T, Mlcek J, Kramarova D, Sengee Z. Antioxidant activity and selected nutritional values of plums (Prunus domestica L.) typical of the White Carpathian Mountains. Sci. Hortic. [Internet]. 2009; 122(4):545-549. doi: https://doi.org/ct4gcb DOI: https://doi.org/10.1016/j.scienta.2009.06.036
Rupasinghe HPV, Jayasankar S, Lay W. Variation in total phenolics and antioxidant capacity among European plum genotypes. Sci. Hortic. [Internet]. 2006; 108(3):243-246. doi: https://doi.org/fxhfnd DOI: https://doi.org/10.1016/j.scienta.2006.01.020
Gonzáles-García E, Marina ML, Concepción García M. Plum (Prunus domestica L.) by-Product as a New and Cheap Source of Bioactive Peptides: Extraction Method and Peptites Characterization. J. Funct. Foods. [Internet]. 2014; 11:428-437. doi: https://doi.org/f6t5vc DOI: https://doi.org/10.1016/j.jff.2014.10.020
Murathan ZT, Arslan M, Erbil N. Analyzing Biological Properties of Some Plum Genotypes Grown in Turkey. Int. J. Fruit Sci. [Internet]. 2020; 20(3):S1729-S1740. doi: https://doi.org/qg58 DOI: https://doi.org/10.1080/15538362.2020.1830917
Blois MS. Antioxidant determination by the use of a stable free radical. Nature. [Internet]. 1958; 181:1199- 1200. doi: https://doi.org/bdq98t DOI: https://doi.org/10.1038/1811199a0
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. [Internet]. 1968; 25(1):192-205. doi: https://doi.org/csbsfm DOI: https://doi.org/10.1016/0003-2697(68)90092-4
Spanos GA, Wrolstad RE. Phenolics of Apple, Pear, and White Grape Juices and Their Changes with Processing and Storage - a Review. J. Agric Food Chem. [Internet]. 1992; 40(9):1478-1487. doi: https://doi.org/b27dmg DOI: https://doi.org/10.1021/jf00021a002
Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin JC, Bailleul F, Trotin F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. [Internet]. 2000; 72(1-2):35-42. doi: https://doi.org/ft249d DOI: https://doi.org/10.1016/S0378-8741(00)00196-3
Garzón GA, Wrolstad RE. The stability of pelargonidin- based anthocyanins at varying water activity. Food Chem. [Internet]. 2001; 75(2):185-196. doi: https://doi.org/bdgkq5 DOI: https://doi.org/10.1016/S0308-8146(01)00196-0
Gonçalves B, Oliveira I, Bacelar E, Morais MC, Aires A, Cosme F, Ventura-Cardoso J, Anjos R, Pinto T. Aromas and Flavours of Fruits. In: Vilela A, editor. Generation of Aromas and Flavours. Rijeka: IntechOpen. 2018; 2:9-31. https://doi.org/gpx6qr DOI: https://doi.org/10.5772/intechopen.76231
Sánchez-Rodríguez L, Ali NS, Cano-Lamadrid M, Noguera- Artiaga L, Lipan L, Carbonell-Barrachina ÁA, Sendra E. - Flavors and Aromas. In: Yahia EM, editor. Postharvest Physiology and Biochemistry of Fruits and Vegetables. Chapter 18. Cambridge, Inglaterra: Woodhead Publishing; 2019. p. 385-404. doi: https://doi.org/gjvq89 DOI: https://doi.org/10.1016/B978-0-12-813278-4.00019-1
Aragüez I, Valpuesta-Fernández V. Metabolic engineering of aroma components in fruits. Biotechnol. J. [Internet]. 2013; 8(10):1144-1158. doi: https://doi.org/f2dv49 DOI: https://doi.org/10.1002/biot.201300113
Du M, Zhu Y, Nan H, Zhou Y, Pan X. Regulation of sugar metabolism in fruits. Sci. Hortic. [Internet]. 2024; 326:112712. doi: https://doi.org/g8rk6p DOI: https://doi.org/10.1016/j.scienta.2023.112712
Pashaei M, Hassanpour H. Phenolic, amino acids, and fatty acids profiles and the nutritional properties in the fresh and dried fruits of black rosehip (Rosa pimpinellifolia L.). Sci. Rep. [Internet]. 2024; 14(1):19665. doi: https://doi.org/qg6b DOI: https://doi.org/10.1038/s41598-024-70574-5
Kafkas E, Kosar M, Türemis N, Baser KHC. Analysis of sugars, organic acids and vitamin C contents of blackberry genotypes from Turkey. Food Chem. [Internet]. 2006; 97(4):732-736. doi: https://doi.org/c8jjfp DOI: https://doi.org/10.1016/j.foodchem.2005.09.023
Celik F, Gundogdu M, Alp S, Muradoglu F, Ercisli S, Gecer MK, Canan I. Determination of Phenolic Compounds, Antioxidant Capacity and Organic Acids Contents of Prunus domestica L., Prunus cerasifera Ehrh. and Prunus spinosa L. Fruits by HPLC. Acta Chromatogr. [Internet]. 2017; 29(4):507-510. doi: https://doi.org/qg6c DOI: https://doi.org/10.1556/1326.2017.00327
Sahoo A, Sarkar S, Lal B, Kumawat P, Sharma S, De K. Utilization of Fruit and Vegetables Waste as an Alternative Feed Resource for Sustainable and Eco-Friendly Sheep Farming. Waste Manag. [Internet]. 2021; 128:232-242. doi: https://doi.org/gj3gs DOI: https://doi.org/10.1016/j.wasman.2021.04.050