Effects of N–acetyl cysteine on serum podocalyxin and pentraxin levels in an experimental lower extremity ischemia–reperfusion injury model
Abstract
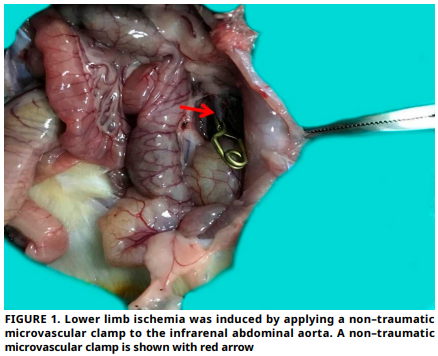
Ischemia–reperfusion injury causes oxidative stress and inflammation, leading to skeletal muscle damage. This study investigates the role of N–acetylcysteine in modulating oxidative stress and inflammatory biomarkers specifically Podocalyxin and Pentraxin 3 in a rat model of lower extremity Ischemia–reperfusion injury. An experimental, controlled animal study conducted at the Experimental Research Center of Firat University. Twenty–four female Sprague–Dawley rats were allocated into four groups: control, sham, Ischemia–reperfusion, and Ischemia–reperfusion treated with N–acetylcysteine. Ischemia was induced by clamping the infrarenal abdominal aorta for 120 min, followed by 120 min of reperfusion. A single dose of N–acetylcysteine (150 mg·kg-1, i.p.) was administered at the onset of reperfusion in the treatment group. Levels of serum Total Oxidative Status and Total Antioxidant Status, as well as the expression of Podocalyxin and Pentraxin 3 in tissue, were evaluated. A significant increase in Oxidative Status levels and a significant decrease in Antioxidant Status levels were observed in the Ischemia–reperfusion group compared to the control group. After administering N–acetylcysteine, there was a significant decrease in Oxidative Status levels and a significant increase in Antioxidant Status levels when compared to the Ischemia–reperfusion group. Histological evaluation showed that N–acetylcysteine reduced edema, hemorrhage, and overall tissue injury scores. Immunohistochemical analyses revealed increased Podocalyxin and Pentraxin 3 expression in Ischemia– reperfusion group tissues, which was notably diminished in the N–acetylcysteine – treated group. N–acetylcysteine demonstrated protective effects against Ischemia–reperfusion–induced oxidative and inflammatory damage in skeletal muscle by reducing serum and tissue levels of Podocalyxin and Pentraxin 3. These findings suggest its therapeutic potential in mitigating Ischemia– reperfusion injury and highlight Podocalyxin and Pentraxin 3 as promising biomarkers for tissue damage and treatment monitoring.
Downloads
References
Ding S, Nie Y, Zhang X, Liu X, Wang C, Yuan R, Chen K, Zhu Q, Cai S, Fang Y, Chen Y, Mo D. The SNPs in myoD gene from normal muscle developing individuals have no effect on muscle mass. BMC Genet. [Internet]. 2019; 20(1):72. doi: https://doi.org/qjfb DOI: https://doi.org/10.1186/s12863-019-0772-6
Zhang Y, Li H, Wang M, Meng G, Wang Z, Deng J, Wang M, Zhang Q, Yang S, Jiang H. Vagus nerve stimulation attenuates acute skeletal muscle injury induced by ischemia– reperfusion in rats. Oxid. Med. Cell. Longev. [Internet]. 2019; 2019:9208949. doi: https://doi.org/gp5nz6 DOI: https://doi.org/10.1155/2019/9208949
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int. Rev. Cel. Mol. Biol. [Internet]. 2012; 298:229–317. doi: https://doi.org/f35x5z
Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin—the major sialoprotein of the renal glomerular epithelial cell. J. Cell Biol. [Internet]. 1984; 98(4):1591–1596. doi: https://doi.org/bsnrk3 DOI: https://doi.org/10.1083/jcb.98.4.1591
Larrucea S, Butta N, Arias–Salgado EG, Alonso–Martin S, Ayuso MS, Parrilla R. Expression of podocalyxin enhances the adherence, migration, and intercel – lular communication of cells. Exp. Cell Res. [Internet]. 2008; 314(10):2004–2015. doi: https://doi.org/dnkf8x DOI: https://doi.org/10.1016/j.yexcr.2008.03.009
El–Ashmawy HM, Selim FO, Hosny TAM, Almassry HN. Association of serum podocalyxin levels with peripheral arterial disease in patients with type 2 diabetes. J. Diabetes Complicat. [Internet]. 2019; 33(7):495–499. doi: https://doi.org/qjfc DOI: https://doi.org/10.1016/j.jdiacomp.2019.04.003
Cieślik P, Hrycek A. Long pentraxin 3 (PTX3) in the light of its structure, mechanism of action and clinical implications. Autoimmunity [Internet]. 2012; 45(2):119–128. doi: https://doi.org/cc8cwh DOI: https://doi.org/10.3109/08916934.2011.611549
Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3(PTX 3): an endogenous modulator of the inflammatory response. Mediators Inflamm. [Internet]. 2012; 2012:920517. doi: https://doi.org/f99zvs DOI: https://doi.org/10.1155/2012/920517
Bottazzi B, Inforzato A, Messa M, Barbagallo M, Magrini E, Garlanda C, Mantovani A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. [Internet]. 2016;64(6):1416–1427. doi: https://doi.org/f8mr9m DOI: https://doi.org/10.1016/j.jhep.2016.02.029
Abedini–Bajgiran F, Khazaei–Koohpar Z, Salehzadeh A. Effects of N–Acetylcysteine supplementation on oxidative stress and expression of apoptosis–related genes in testicular tissue of rats exposed to Lead. Biol. Trace Elem. Res. [Internet]. 2023;201(5):2407–2415. doi: https://doi.org/qjfd DOI: https://doi.org/10.1007/s12011-022-03325-0
Saricaoglu F, Dal D, Salman AE, Atay OA, Doral MN, Salman MA, Kilinç K, Aypar U. Effect of low–dose N–acetyl–cysteine infusion on tourniquet–induced ischaemia–reperfusion injury in arthroscopic knee surgery. Acta Anaesthesiol. Scand. [Internet]. 2005; 49(6):847–851. doi: https://doi.org/c34f7w DOI: https://doi.org/10.1111/j.1399-6576.2005.00722.x
Debruin EJ, Hughes MR, Sina C, Lu A, Cait J, Jian Z, Lopez M, Lo B, Abraham T, McNagny KM. Podocalyxin regulates murine lung vascular permeability by altering endothelial cell adhesion. PLoS One [Internet]. 2014; 9(12):e116613. doi: https://doi.org/qjfh DOI: https://doi.org/10.1371/journal.pone.0116613
Chen Q, Wang Y, Li Y, Zhao M, Nie G. Serum podocalyxin is significantly increased in early–onset preeclampsia and may represent a novel marker of maternal endothelial cell dysfunction. J. Hypertens. [Internet]. 2017; 35(11):2287–2294. doi: https://doi.org/g4g8wh DOI: https://doi.org/10.1097/HJH.0000000000001461
Yorganci A, Halici–Ozturk F, Hancerliogullari N, Çandar T, Caglar AT, Ozgu–Erdinc AS. The role of serum podocalyxin levels in recurrent pregnancy loss. Eur. J. Obstet. Gynecol. Reprod. Biol. [Internet]. 2021; 260:114–117. doi: https://doi.org/qjfj DOI: https://doi.org/10.1016/j.ejogrb.2021.03.021
Du Clos TW, Mold C. Pentraxins (CRP, SAP) in the process of complement activation and clearance of apoptotic bodies through Fcγ receptors. Curr. Opin. Organ. Transplant. [Internet]. 2011; 16(1):15–20. doi: https://doi.org/c2n6z7 DOI: https://doi.org/10.1097/MOT.0b013e32834253c7
Zhu H, Cui D, Liu K, Wang L, Huang L, Li J. Long pentraxin PTX3 attenuates ischemia reperfusion injury in a cardiac transplantation model. Transpl. Int. [Internet]. 2014; 27(1):87–95. doi: https://doi.org/f5k7pk DOI: https://doi.org/10.1111/tri.12197
Souza DG, Amaral FA, Fagundes CT, Coelho FM, Arantes RM, Sousa LP, Matzuk MM, Garlanda C, Mantovani A, Dias AA, Teixeira MM. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am. J. Pathol. [Internet]. 2009;174(4):1309–1318. doi: https://doi.org/dmx3f3 DOI: https://doi.org/10.2353/ajpath.2009.080240
Lech M, Römmele C, Gröbmayr R, Eka–Susanti H, Kulkarni OP, Wang S, Gröne HJ, Uhl B, Reichel C, Krombach F, Garlanda C, Mantovani A, Anders HJ. Endogenous and exogenous pentraxin–3 limits postischemic acute and chronic kidney injury. Kidney Int. [Internet]. 2013; 83(4):647–661. doi: https://doi.org/qjfk DOI: https://doi.org/10.1038/ki.2012.463
Shimizu T, Suzuki S, Sato A, Nakamura Y, Ikeda K, Saitoh S, Misaka S, Shishido T, Kubota I, Takeishi Y. Cardio–protective effects of pentraxin 3 produced from bone marrow–derived cells against ischemia/reperfusion injury. J. Mol. Cell. Cardiol. [Internet]. 2015 ;89:306–313. doi: https://doi.org/f75rmq DOI: https://doi.org/10.1016/j.yjmcc.2015.10.013
Hortu I, Ilgen O, Sahin C, Akdemir A, Yigitturk G, Erbas O. Losartan ameliorates ovarian ischaemia/reperfusion injury in rats: an experimental study. J. Obstet. Gynaecol. [Internet]. 2020; 40(8):1148–1154. doi: https://doi.org/qjfm DOI: https://doi.org/10.1080/01443615.2019.1701639