Physicochemical, antioxidant and fatty acid quality in Mexican hairless pork fed with Moringa oleifera and Brosimum alicastrum
Abstract
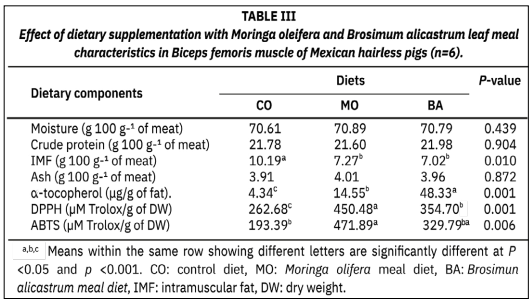
This study aimed to evaluate the effects of diets with Moringa oleifera and Brosimum alicastrum leaf meal on the physicochemical characteristics, antioxidant capacity and fatty acid composition of meat from Mexican Hairless Pigs. Eighteen (18) Mexican Hairless Pigs pigs were used in the study. The pigs were randomly divided into three dietary groups: control diet and two experimental diets supplemented with 10 % Moringa oleifera and Brosimum alicastrum leaf meal, respectively. The results indicated that intramuscular fat was higher (P < 0.05) in meat from Mexican Hairless Pigs fed control diet. Meat from Mexican Hairless Pigs fed Brosimum alicastrum diets presented higher (P < 0.001) α-tocopherol (48.33 μg/g of fat). The 2,2-diphenyl- 1-picrylhydrazyl concentration was higher (P < 0.05) in the meat of pigs supplemented with Moringa oleifera (450.48 μM Trolox/g of DW). Pigs fed Brosimum alicastrum leaf meal were characterized by a higher content of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) in their meat (P < 0.05) (471.89 μM Trolox/g of DW). Regarding the fatty acid composition, meat from Mexican Hairless Pigs fed Moringa oleifera and Brosimum alicastrum presented higher values (P < 0.001; P < 0.05) of saturated fatty acids and atherogenic and thrombogenic indices. In contrast, the concentrations of monounsaturated fatty acids and polyunsaturated fatty acids, unsaturated fatty acids, the polyunsaturated fatty acids/saturated fatty acids ratio and the monounsaturated fatty acids/saturated fatty acids ratio were higher (P< 0.001; P < 0.05) in meat from the control group. The nutritive value index did not differ (P > 0.05) between treatments. It is concluded that Moringa oleifera and Brosimum alicastrum leaf meal could be used as an Mexican Hairless Pigs feed resource to reduce intramuscular fat in meat.
Downloads
References
Wang L , Huang Y, W ang Y, Shan T. Eff ects o f polyunsaturared fatty acids supplementation on the meat quality of pigs: A meta-analysis. Front. Nutr. [Internet]. 2021; 8:746765. doi: https://doi.org/gnrptm DOI: https://doi.org/10.3389/fnut.2021.746765
Lebret B, Ecolan P, Bonhomme N, Méteau K, Prunier A. Influence of production system in local and conventional pig breeds on stress indicators at slaughter, muscle and meat traits and pork eating quality. Animal. [Internet]. 2015; 9(8):1404-1413. doi: https://doi.org/gtd7v3 DOI: https://doi.org/10.1017/S1751731115000609
Hernández A, García CA, García AM, Ortíz JR, Sierra ÁC, Morales S. The production system of hairless creole pig (Cerdo Pelon Mexicano) in the Peninsula of Yucatan. Nova Scientia. [Internet]. 2020, 24(12):1–21. doi: https://doi.org/qhxz
Abdelnour SA, El-Hack MEA, Ragni M. The efficacy of high- protein tropical forages as alternative protein sources for chickens: A review. Agriculture. [Internet]. 2018; 8(6):86. doi: https://doi.org/qhx4 DOI: https://doi.org/10.3390/agriculture8060086
Ekpo JS, Okon UM. Performance and lipid profile of growing pigs fed Vernonia amygdalina and Jathropha tanjorensis leaf meal supplementation. Livest. Res. Rural Dev. [Internet]. 2022 [Accesed 15 Nov 2024]; 34(12):111. Available in: https://goo.su/B33VpwG
Zeng Z, Jiang JJ, Yu J, Mao XB, Yu B, Chen DW. Effect of dietary supplementation with mulberry (Morus alba L.) leaves on the growth performance, meat quality and antioxidative capacity of finishing pigs. J. Integr. Agric. [Internet]. 2019; 18(1):143–151. doi: https://doi.org/qhx5 DOI: https://doi.org/10.1016/S2095-3119(18)62072-6
Falowo AB, Mukumbo F E, Idamokoro EM Lorenzo JM, Afolayan AJ, Muchenje V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. [Internet]. 2018; 106:317-334. doi: https://doi.org/gdfkpk DOI: https://doi.org/10.1016/j.foodres.2017.12.079
Faustin E, Sarmiento L, Sandoval C, Segura J, Caamal JA. Male layer chicken’s response to dietary Moringa oleifera meal in a tropical climate. Animals. [Internet]. 2022; 12(14):1843. doi: https://doi.org/qhx6 DOI: https://doi.org/10.3390/ani12141843
Abdel SM, Hassan EH. Effects of dietary inclusion of Moringa oleifera leaf meal on nutrient digestibility, rumen fermentation, ruminal enzyme activities and growth performance of buffalo calves. Saudi J. Biol. Sci. [Internet]. 2021; 28(8):4430-4436. doi: https://doi.org/qhx7 DOI: https://doi.org/10.1016/j.sjbs.2021.04.037
Wankhede SD, Dutta N, Tambe MB, Kaur N, Pattanaik AK. Effect of dietary inclusion of Moringa oleifera foliage on nutrient metabolism, metabolic profile, immunity and growth performance of goat kids. Emerg. Anim. Species.
Santillán A, González C, Bautista J, Huicab ZG, Escobar J, Larqué A. Brosimum Alicastrum Swartz as an alternative for the productive reconversion of agrosilvopastoral areas in Campeche. Rev. Mex. Cienc. Forest. [Internet]. 2020; 11(61):51-69 doi: https://doi.org/qhx9 DOI: https://doi.org/10.29298/rmcf.v11i61.722
Cob-Garma ME, Sarmiento-Franco LA. Effect of dietary inclusion of Brosimum alicastrum swartz leaf meal on diarrhea control in piglets. Trop. Subtrop. Agroecosyst. [Internet]. 2019; 22(1):163–167. doi: https://doi.org/qhzb DOI: https://doi.org/10.56369/tsaes.2616
Moo-Huchin VM, Canto-Pinto JC, Cuevas-Glory LF, Sauri- Duch E, Pérez-Pacheco E, Betancur-Ancona D. Effect of extraction solvent on the phenolic compounds content and antioxidant activity of Ramon nut (Brosimum alicastrum). Chem. Pap. [Internet]. 2019; 73:1647-1657. doi: https://doi.org/pgst DOI: https://doi.org/10.1007/s11696-019-00716-x
Olvera-Aguirre G, Mendoza-Taco MM, Moo-Huchin VM, Lee-Rangel HA, Roque-Jiménez JA, Gómez-Vázquez A, Dzib-Cauich DA, Chay-Canul AJ. Effect of extraction type on bioactive compounds and antioxidant activity of Moringa oleifera Lam. leaves. Agriculture. [Internet]. 2022; 12(9):1462. doi: https://doi.org/qhzc DOI: https://doi.org/10.3390/agriculture12091462
National Research Council (NRC). Nutrient Requirements for Swine. 10th ed. Washington, DC: The National Academies Press. 1998.
Hanson SWF, Olley J. Application of the bligh and dyer method of lipid extraction to tissue homogenates. Biochem, J. [Internet]. 1963 [cited 15 Jan 2024]; 89:101-102.
Morrison WR, Smith LM. Preparation of fatty acid methyl [26] Tomovic VM, Ševic R, Jokanovic M, Šojic B, Škaljac S, Tasic esters and dimethyl acetals from lipids with boron T, Ikonic P, Polak ML, Polak T, Demšar L. Quality traits fluoride-methanol. J. Lipid Res. [Internet]. 1964; 5:600-608. doi: https://doi.org/pv9m DOI: https://doi.org/10.1016/S0022-2275(20)40190-7
Dzib-Cauich D, Lemus-Flores C, Bugarín-Prado JO, Ayala- Valdovinos MA, Moo-Huchin VM. Perfil de ácidos grasos en músculo Longissimus dorsi y expresión de genes asociados con metabolismo lipídico en cerdos pelón mexicanos y cerdos Landrace-Yorkshire. Livest. Res. Rural Dev. [Internet]. 2020 [Accesed 10 Dec 2024]; 32(7):115. Available in: https://goo.su/iznOWr6
Ulbricht TLV, Southgate DAT. Coronary heart disease: seven dietary factors. Lancet. [Internet]. 1991; 338(8773):985-992. doi: https://doi.org/dghp85 DOI: https://doi.org/10.1016/0140-6736(91)91846-M
Cañeque V, Díaz MT, Álvarez I, Lauzurica S, Pérez C, De la Fuente J. The influences of carcass weight and depot on the fatty acid composition of fats of suckling Manchego lambs. Meat Sci. [Internet]. 2005; 70(2):373-379. doi: https://doi.org/crbqmm DOI: https://doi.org/10.1016/j.meatsci.2005.02.003
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. [Internet]. 1995; 28(1):25-30. doi: https://doi.org/fgbszk DOI: https://doi.org/10.1016/S0023-6438(95)80008-5
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. [Internet]. 1993; 84(4):407-412. doi: https://doi.org/gp5s4n DOI: https://doi.org/10.1042/cs0840407
Pieszka M, Szczurek P, Bederska-Łojewska D, Migdał W, Pieszka M, Gogol P, Jagusiak W. The effect of dietary supplementation with dried fruit and vegetable pomaces on production parameters and meat quality in fattening pigs. Meat Sci. [Internet]. 2017; 126:1-10. doi: https://doi.org/f9t5x3 DOI: https://doi.org/10.1016/j.meatsci.2016.11.016
Chu GM, Park BK. Effects of fermented carrot by-product diets on growth performances, carcass characteristics and meat quality in fattening pigs. Acta Agric. Scand. A Anim. Sci. [Internet]. 2023; 72(1–2):40-48. doi: https://doi.org/qhzt DOI: https://doi.org/10.1080/09064702.2023.2191611
Baerley VR, Ferreira CC, Vinhas LC, Domingues CE, Bonin MDN. dos Santos G, dos Santos GT, Ayardes GK, Chaves AL, de Melo ES, de Sousa TFF, de Godoy C, da Silva AA, Bernardo P. Antioxidant action of yerba mate on carcass and meat characteristics and fatty acid profile in meat and fat of lambs finished in tropical pastures. Trop. Anim. Health Prod. [Internet]. 2023; 55:109. doi: https://doi.org/qhzv DOI: https://doi.org/10.1007/s11250-023-03521-7
Tomović VM, Šević R, Jokanović M, Šojić B, Škaljac S, Tasić T, Ikonić P, Polak ML, Polak T, Demšar L. Quality traits of longissimus lumborum muscle from White Mangalica, Duroc_White Mangalica and Large White pigs reared under intensive conditions and slaughtered at 150 kg live weight: a comparative study. Arch. Anim. Breed. [Internet]. 2016; 59:401-415. doi: https://doi.org/bq5h DOI: https://doi.org/10.5194/aab-59-401-2016
Kim GW, Kim HY. Comparison of physicochemical properties between standard and sow pork. Korean J. Food Sci. Anim. Resour. [Internet]. 2018; 38(5):1120- 1130. doi: https://doi.org/qhzx DOI: https://doi.org/10.5851/kosfa.2018.e45
Rey AI, Daza A, López-Carrasco C, López-Bote CJ. Feeding Iberian pigs with acorns and grass in either free-range or confinement affects the carcass characteristics and fatty acids and tocopherols accumulation in Longissimus dorsi muscle and backfat. Meat Sci. [Internet]. 2006; 73(1):66-74. doi: https://doi.org/c54vfv DOI: https://doi.org/10.1016/j.meatsci.2005.10.018
Qwele K, Hugo A, Oyedemi SO, Moyo B, Masika PJ, Muchenje V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves sunflower cake and grass hay. Meat Sci. [Internet]. 2013; 93(3):455-462. doi: https://doi.org/f4kwzk DOI: https://doi.org/10.1016/j.meatsci.2012.11.009
Luo Y, Wang B, Liu C, Su R, Hou Y, Yao D, Zhao L, Su L, Jin Y. Meat quality, fatty acids, volatile compounds, and antioxidant properties of lambs fed pasture versus mixed diet. Food Sci. Nutr. [Internet]. 2019; 7(9):2796-2805. doi: https://doi.org/qhzz DOI: https://doi.org/10.1002/fsn3.1039
Quander-Stoll N, Bautze D, Zollitsch W, Leiber F, Früh B. Effects of 100% organic feeding on performance, carcass composition and fat quality of fattening pigs. Biol. Agric. Hortic. [Internet]. 2022; 38(4):271-284. doi: https://doi.org/qhz2 DOI: https://doi.org/10.1080/01448765.2022.2119889
Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in c a r d i o va s c u l a r p r e v e n t i o n . B io c h e m . P h a r m a c ol . [Internet]. 2009; 77(6):937-946. doi: https://doi.org/b6tqt5 DOI: https://doi.org/10.1016/j.bcp.2008.10.020
Chernukha I, Kotenkova E, Pchelkina V, Ilyin N, Utyanov D, Kasimova T, Surzhik A, Fedulova L. Pork fat and meat: A balance between consumer expectations and nutrient composition of four pig breeds. Foods. [Internet]. 2023; 12(4):690. doi: https://doi.org/qhz3 DOI: https://doi.org/10.3390/foods12040690
Güngör ÖF, Özbeyaz C, Ünal N, Akçapınar H. The evaluation of the genotype and slaughter weight effect on meat quality and fatty acid profile from two native sheep. Trop. Anim. Health Prod. [Internet]. 2023; 55(2):116. doi: https://doi.org/qhz4 DOI: https://doi.org/10.1007/s11250-023-03523-5
Zhang T, Si B, Tu Y, Cui K, Zhou C, Diao Q. Effect of including different levels of moringa (Moringa oleifera) leaf meal in the diet of finishing pigs: Performance, pork quality, fatty acid composition, and amino acid profile. Czech J. Anim. Sci. [Internet]. 2019; 64(3):141-149. doi: https://doi.org/qhz7 DOI: https://doi.org/10.17221/204/2018-CJAS
Schwingshackl L, Hoffmann G. Dietary fatty acids in the secondary prevention of coronary heart disease: A systematic review, meta-analysis and meta-regression. BMJ Open. [Internet]. 2014: 4(4):e004487. doi: https://doi.org/f6m6tc DOI: https://doi.org/10.1136/bmjopen-2013-004487