Evaluación histopatológica de los efectos de la aplicación sistémica de Ranelate de Estroncio en la curación ósea tras injerto de defecto tibial
Resumen
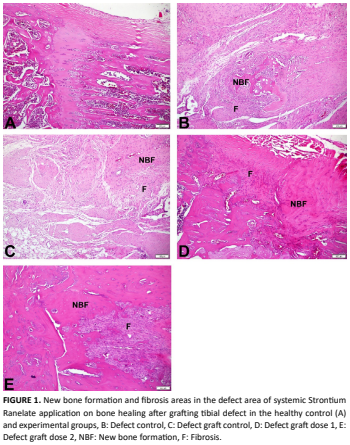
Este estudio evaluó los efectos de diferentes dosis de ranelato de estroncio combinadas con un injerto óseo xenogénico desproteinizado de origen bovino sobre la cicatrización ósea en un modelo de defecto en ratas. Treinta y cinco ratas Sprague Dawley hembra fueron asignadas aleatoriamente a cinco grupos (n = 7). El grupo control sano no recibió tratamiento. En los demás grupos se creó un defecto estandarizado de 4 mm × 4 mm en la región metafisaria de la tibia. El grupo con defecto no recibió tratamiento adicional. En el grupo defecto–injerto, el defecto se rellenó con el injerto óseo. En los grupos defecto– injerto + estroncio, además del injerto, se administró ranelato de estroncio por vía oral a dosis de 450 mg/kg o 900 mg/kg, tres veces por semana durante ocho semanas. Al final del período experimental, todas las ratas fueron eutanasiadas y los tejidos óseos se procesaron para análisis histológico. La normalidad de los datos se evaluó mediante las pruebas de Shapiro–Wilk y Kolmogorov–Smirnov. Dado que los datos no siguieron una distribución normal, las comparaciones entre grupos se realizaron con la prueba de Kruskal–Wallis y la prueba U de Mann–Whitney como análisis post hoc. Los valores medios del defecto horizontal fueron 0 en el grupo control sano, 716,86 en el grupo con defecto, 658,57 en el grupo defecto–injerto, 604,57 en el grupo defecto–injerto dosis 1 y 598,86 en el grupo dosis 2. Los valores medios verticales fueron 0 en el grupo sano, 575,14 en el grupo con defecto, 596,43 en el grupo defecto– injerto, 569 en el grupo dosis 1 y 503,29 en el grupo dosis 2. En conclusión, el ranelato de estroncio mostró un efecto positivo sobre la cicatrización ósea, especialmente cuando se combinó con injerto, observándose una diferencia significativa entre el grupo con defecto y el grupo de dosis alta.
Descargas
Citas
Nayak VV, Goncalves JAKQ, Mirsky NA, Arakelians ARL, Bergamo ETP, Torroni A, Boczar D, Coelho PG, Witek L. Comparison of Bovine and Porcine Collagen Membranes for Potential Applications in Guided Bone Regeneration: An In Vivo Pre-Clinical Evaluation. J. Biomed. Mater. Res. B Appl. Biomater. [Internet]. 2025; 113(10):e35651. doi: https://doi.org/qk93 DOI: https://doi.org/10.1002/jbm.b.35651
Chen O, Hu Y, Xu B, Xu W. Impact of Combining Alfacalcidol With Proximal Femoral Nail Antirotation on Bone Mineral Density, Serum Bone Metabolites, and Inflammatory Markers in Elderly Patients With Osteoporotic Intertrochanteric Fractures. Ann. Ital. Chir. [Internet]. 2025; 96(9):1180-1189. doi: https://doi.org/qk94 DOI: https://doi.org/10.62713/aic.4168
Mihali SG, Talpoș Ș, Popa M, Loloș D, Bonomo S, Hajaj T. Diagnostic and Clinical Outcomes of Three Regenerative Strategies for Alveolar Bone Defects: A Comparative Study Using CBCT and ISQ. Diagnostics (Basel). [Internet]. 2025; 15(16):2078. doi: https://doi.org/qk95 DOI: https://doi.org/10.3390/diagnostics15162078
Ahmed Omar N, Roque J, Bergeaut C, Bidault L, Amédée J, Letourneur D, Fricain JC, Fenelon M. Challenges and limitations in developing of a new maxillary standardized rat alveolar bone defect model to study bone regenerative approaches in oral and maxillofacial surgery. Front. Bioeng. Biotechnol. [Internet]. 2025; 13:1494352. doi: https://doi.org/qk96 DOI: https://doi.org/10.3389/fbioe.2025.1494352
Freire GCB, Gonçalves PF, Pimentel SP, Nociti Júnior FH, Casati MZ, Gurgel BCV. Influence of residual buccal bone thickness in dehiscence defects on osseointegrated dental implants in healed sites: an experimental in vivo study. Braz. Oral. Res. [Internet]. 2025; 39:e079. doi: https://doi.org/qk97 DOI: https://doi.org/10.1590/1807-3107bor-2025.vol39.079
Wang HL, Hazrati P, Calatrava J, Saleh MS, Alrmali AE. Long-term clinical outcomes of periodontal regeneration of intrabony defects: A systematic review and meta-analysis. Periodontol. 2000. [Internet]. 2025; 10.1111:70002. doi: https://doi.org/qk98 DOI: https://doi.org/10.1111/prd.70002
Matassi F , Nistri L, Chicon Paez D, Innocenti M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011 [cited 20 Nov 2025]; 8(1):21-24. PMID: 22461799. Available in: https://goo.su/u6L35
Lin K, Liu P, Wei L, Zou Z, Zhang W, Qian Y, Shen Y, Chang J. Strontium substituted hydroxyapatite porous microspheres: Surfactant-free hydrothermal synthesis, enhanced biological response and sustained drug release. Chem. Eng. J. [Internet]. 2013; 222:49-59. doi: https://doi.org/f4znn4 DOI: https://doi.org/10.1016/j.cej.2013.02.037
Goldhahn J, Scheele WH, Mitlak BH, Abadie E, Aspenberg P, Augat P, Brandi ML, Burlet N, Chines A, Delmas PD, Dupin-Roger I, Ethgen D, Hanson B, Hartl F, Kanis JA, Kewalramani R, Laslop A, Marsh D, Ormarsdottir S, Rizzoli R, Santora A, Schmidmaier G, Wagener M, Reginster JY. Clinical evaluation of medicinal products for acceleration of fracture healing in patients with osteoporosis. Bone. [Internet]. 2008; 43(2):343-347. doi: https://doi.org/fvthqp
Liu HY, Wu ATH, Tsai CY, Chou KR, Zeng R, Wang MF, Chang WC, Hwang SM, Su CH, Deng WP. The balance between adipogenesis and osteogenesis in bone regeneration by platelet-rich plasma for age-related osteoporosis. Biomaterials. [Internet]. 2011; 32(28):6773-6780. doi: https://doi.org/bsm5ht DOI: https://doi.org/10.1016/j.biomaterials.2011.05.080
Cao L, Liu G, Gan Y, Fan Q, Yang F, Zhang X, Tang T, Dai K. The use of autologous enriched bone marrow MSCs to enhance osteoporotic bone defect repair in long-term estrogen deficient goats. Biomaterials. [Internet]. 2012; 33(10):5076-5084. doi: https://doi.org/f3ztj8 DOI: https://doi.org/10.1016/j.biomaterials.2012.03.069
Cho SW, Sun HJ, Yang JY, Jung JY, Choi HJ, An JH, Kim SW, Kim SY, Park KJ, Shin CS. Human adipose tissue-derived stromal cell therapy prevents bone loss in ovariectomized nude mouse. Tissue Eng Part A. [Internet]. 2012; 18(9- 10):1067-1078. doi: https://doi.org/fzcs7r DOI: https://doi.org/10.1089/ten.tea.2011.0355
Marie PJ, Felsenberg D, Brandi ML. How strontium ranelate, via opposite effects on bone resorption and formation, prevents osteoporosis. Osteoporos. Int. [Internet]. 2011; 22(6):1659-1667. doi: https://doi.org/dknkm4 DOI: https://doi.org/10.1007/s00198-010-1369-0
Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. [Internet]. 2008; 42(1):129-138. doi: https://doi.org/d9kckw DOI: https://doi.org/10.1016/j.bone.2007.08.043
Baron R, Tsouderos Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur. J. Pharmacol. [Internet]. 2002; 450(1):11-17. doi: https://doi.org/dqjc55 DOI: https://doi.org/10.1016/S0014-2999(02)02040-X
Hulsart-Billström G, Xia W, Pankotai E, Weszl M, Carlsson E, Forster-Horváth C, Larsson S, Engqvist H, Lacza Z. Osteogenic potential of Sr-doped calcium phosphate hollow spheres in vitro and in vivo. J. Biomed. Mater. Res. A. 2013; 101A(8):2322-2331. doi: https://doi.org/f22kfs DOI: https://doi.org/10.1002/jbm.a.34526
Baier M, Staudt P, Klein R, Sommer U, Wenz R, Grafe I, Meeder PJ, Nawroth PP, Kasperk C. Strontium enhances osseointegration of calcium phosphate cement: a histomorphometric pilot study in ovariectomized rats. J. Orthop. Surg. Res. [Internet]. 2013; 8:16. doi: https://doi.org/f472jr DOI: https://doi.org/10.1186/1749-799X-8-16
Thormann U, Ray S, Sommer U, ElKhassawna T, Rehling T, Hundgeburth M, Henß A, Rohnke M, Janek J, Lips KS, Heiss C, Schlewitz G, Szalay G, Schumacher M, Gelinsky M, Schnettler R, Alt V. Bone formation induced by strontium modified calcium phosphate cement in critical- size metaphyseal fracture defects in ovariectomized rats. Biomaterials. [Internet]. 2013; 34(34):8589-8598. doi: https://doi.org/f49wpg DOI: https://doi.org/10.1016/j.biomaterials.2013.07.036
Neves N, Linhares D, Costa G, Ribeiro CC, Barbosa MA. In vivo and clinical application of strontium-enriched biomaterials for bone regeneration: A systematic review. Bone Jt. Res. [Internet]. 2017; 6(6):366-375. doi: https://doi.org/qmbr DOI: https://doi.org/10.1302/2046-3758.66.BJR-2016-0311.R1
Cardemil C, Elgali I, Xia W, Emanuelsson L, Norlindh B, Omar O, Thomsen P. Strontium-doped calcium phosphate and hydroxyapatite granules promote different inflammatory and bone remodelling responses in normal and ovariectomised rats. PLoS One. [Internet]. 2013; 8(12):e84932. doi: https://doi.org/f22j6g DOI: https://doi.org/10.1371/journal.pone.0084932
Mao L, Xia L, Chang J, Liu J, Jiang L, Wu C, Fang B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. [Internet]. 2017; 61:217-232. doi: https://doi.org/gmv55t DOI: https://doi.org/10.1016/j.actbio.2017.08.015
Caudrillier A, Hurtel-Lemaire AS, Wattel A, Cournarie F, Godin C, Petit L, Petit JP, Terwilliger E, Kamel S, Brown EM, Mentaverri R, Brazier M. Strontium ranelate decreases receptor activator of nuclear factor-ΚB ligand-induced osteoclastic differentiation in vitro: involvement of the calcium-sensing receptor. Mol. Pharmacol. [Internet]. 2010; 78(4):569-576. doi: https://doi.org/ftnjxd DOI: https://doi.org/10.1124/mol.109.063347
Chattopadhyay N, Quinn SJ, Kifor O, Ye C, Brown EM. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. [Internet]. 2007; 74(3):438-447. doi: https://doi.org/d7rq2r DOI: https://doi.org/10.1016/j.bcp.2007.04.020
Atkins GJ, Welldon KJ, Halbout P, Findlay DM. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos. Int. 2009; 20(4):653-664. doi: https://doi.org/c4q8st DOI: https://doi.org/10.1007/s00198-008-0728-6
Zhu LL, Zaidi S, Peng Y, Zhou H, Moonga BS, Blesius A, Dupin-Roger I, Zaidi M, Sun L. Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem. Biophys. Res. Commun. [Internet]. 2007; 355(2):307-311. doi: https://doi.org/fwnkgt DOI: https://doi.org/10.1016/j.bbrc.2007.01.120
Fromigué O, Haÿ E, Barbara A, Marie PJ. Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J. Biol. Chem. [Internet]. 2010; 285(33):25251-25258. doi: https://doi.org/fsjfcw DOI: https://doi.org/10.1074/jbc.M110.110502
Goldhahn J, Scheele WH, Mitlak BH, Abadie E, Aspenberg P, Augat P, Brandi ML, Burlet N, Chines A, Delmas PD, Dupin-Roger I, Ethgen D, Hanson B, Hartl F, Kanis JA, Kewalramani R, Laslop A, Marsh D, Ormarsdottir S, Rizzoli R, Santora A, Schmidmaier G, Wagener M, Reginster JY. Clinical evaluation of medicinal products for acceleration of fracture healing in patients with osteoporosis. Bone. [Internet]. 2008; 43(2):343-347. doi: https://doi.org/fvthqp DOI: https://doi.org/10.1016/j.bone.2008.04.017
Jebahi S , Oudadesse H, el Feki H, Rebai T, Keskes H, Pellen P, El Feki A. Antioxidative/oxidative effects of strontium-doped bioactive glass as bone graft. In vivo assays in ovariectomised rats. J. Appl. Biomed. [Internet]. 2012; 10:195-209. doi: https://doi.org/qmbv DOI: https://doi.org/10.2478/v10136-012-0009-8
Jebahi S, Oudadesse H, Elleuch J, Tounsi S, Keskes H, Pellen P, Rebai T, El Feki A, El Feki H. The potential restorative effects of strontium-doped bioactive glass on bone microarchitecture after estrogen- deficieny induced osteoporosis: physicochemical and histomorphometric analyses. J. Korean Soc. Appl. Biol. Chem. [Internet]. 2013; 56:533-540. doi: https://doi.org/qmbw DOI: https://doi.org/10.1007/s13765-013-3167-9
Li X, Xu CP, Hou YL, Song JQ, Cui Z, Wang SN, Huang L, Zhou CR, Yu B. A novel resorbable strontium-containing α-calcium sulfate hemihydrate bone substitute: a preparation and preliminary study. Biomed. Mater. [Internet]. 2014; 9(4):045010. doi: https://doi.org/qmbx DOI: https://doi.org/10.1088/1748-6041/9/4/045010
Zhang Y , Wei L , Chang J , Miron RJ , Shi B , Yi S , Wu C . Correction: Strontium-incorporated mesoporous bioactive glass scaffolds stimulating in vitro proliferation and differentiation of bone marrow stromal cells and in vivo regeneration of osteoporotic bone defects. J. Mater. Chem. B. [Internet]. 2019; 7(11):1963. doi: https://doi. org/10.1039/c9tb90031d Erratum for: J Mater Chem B. 2013; 1(41):5711-5722. doi: https://doi.org/qmb2 DOI: https://doi.org/10.1039/C3TB21047B
Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. [Internet]. 2015; 3:15005. doi: https://doi.org/gcctz2 DOI: https://doi.org/10.1038/boneres.2015.5
Kyllönen L, D’Este M, Alini M, Eglin D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. [Internet]. 2015; 11:412-434. doi: https://doi.org/gf7c8z DOI: https://doi.org/10.1016/j.actbio.2014.09.006
Yamaguchi M, Weitzmann MN. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. [Internet]. 2012; 359(1- 2):399-407. doi: https://doi.org/c8969x DOI: https://doi.org/10.1007/s11010-011-1034-8
Barbara A, Delannoy P, Denis BG, Marie PJ. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism. [Internet]. 2004; 53(4):532-537. doi: https://doi.org/fwqqb9 DOI: https://doi.org/10.1016/j.metabol.2003.10.022
Takaoka S, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. The Calcium-sensing Receptor (CaR) is involved in strontium ranelate-induced osteoblast differentiation and mineralization. Horm. Metab. Res. [Internet]. 2010; 42(9):627-631. doi: https://doi.org/dthvqq DOI: https://doi.org/10.1055/s-0030-1255091
Almeida MM, Nani EP, Teixeira LN, Peruzzo DC, Joly JC, Napimoga MH, Martinez EF. Strontium ranelate increases osteoblast activity. Tissue Cell. 2016; 48(3):183-188. doi: https://doi.org/qmb3 DOI: https://doi.org/10.1016/j.tice.2016.03.009
Pilmane M, Salma-Ancane K, Loca D, Locs J, Berzina- Cimdina L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C. 2017; 78:1222-1230. doi: https://doi.org/gbnkwd DOI: https://doi.org/10.1016/j.msec.2017.05.042