Análisis comparativo del éxito del aislamiento de Neospora caninum a partir de diversos tejidos fetales: la importancia de las muestras cerebrales. Nota técnica
Resumen
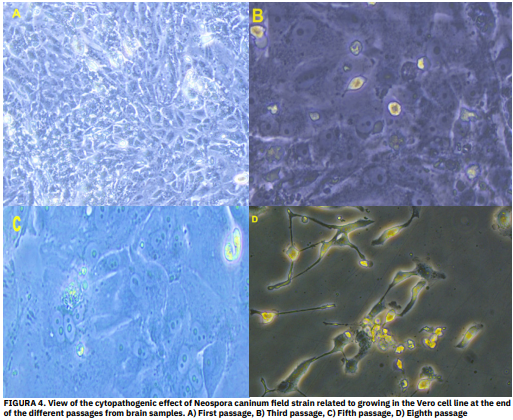
El aislamiento de Neospora caninum a partir de huéspedes infectados naturalmente es crucial para comprender la epidemiología de la neosporosis. Aunque el aislamiento del parásito de varios tejidos fetales, incluido el cerebro, ha sido mencionado, las tasas relativas de éxito del aislamiento a partir de diferentes fuentes no han sido exploradas sistemáticamente. Este estudio examina las tasas de aislamiento de N. caninum a partir de diferentes tejidos fetales, centrándose en la importancia de las muestras cerebrales. Se analizaron los tejidos fetales de 90 bovinos y ovinos infectados naturalmente para detectar la presencia de N. caninum. Las muestras se procesaron con técnicas de aislamiento molecular y de cultivo celular. Se compararon la producción de taquizoítos y los efectos citopatógenos (ECP) en los tejidos después del aislamiento en células Vero. De las 90 muestras, 19 (21,1 %) dieron positivo para N. caninum por PCR y, de estas, 9 (47,36 %) dieron lugar a aislamientos viables. El tejido cerebral de ovejas exhibió una producción de taquizoítos significativamente más alta (1,9 x 107) en comparación con otros tejidos (contenido abomasal, riñón, placenta, hígado y líquido pericárdico). El mayor ECP se observó en el octavo pasaje, al 80%. Las muestras de cerebro parecen ser la fuente más confiable para el aislamiento in vitro de N. caninum a partir de fetos infectados naturalmente. Este hallazgo tiene implicaciones importantes para el diagnóstico y el estudio de la neosporosis ovina.
Descargas
Citas
Benavides J, González-Warleta M, Arteche-Villasol N, Pérez V, Mezo M, Gutiérrez-Expósito D. Ovine neosporosis: The current global situation. Anim. 2022; 12(16): 2074. doi: https://doi.org/pjbg DOI: https://doi.org/10.3390/ani12162074
Reichel MP, Ayanegui-Alcérreca MA, Gondim LFP, Ellis JT. What is the global economic impact of Neospora caninum in cattle-the billion dollar question. Int. J. Parasitol. [Internet]. 2013; 43(2): 133-142. doi: https://doi.org/f4qz3c DOI: https://doi.org/10.1016/j.ijpara.2012.10.022
Zhu Y, Zhu ZF, Yang X, Liu J, Liu Q. Prevalence and associated risk factors of Neospora caninum infection among cattle in mainland China: A systematic review and meta-analysis. Prev. Vet. Med. [Internet]. 2022; 201: 105593. doi: https://doi.org/gsdczw DOI: https://doi.org/10.1016/j.prevetmed.2022.105593
Han FJ, Liu J, Nan HZ, Liu Q. Serological investigation of Neospora caninum in a dairy farm in Beijing. Chin. J. Vet. Med. 2015; 51(12): 50-52.
Fonseca-Martinez BA, Leotti VB, Borba MR, Silva GS, Corbellini LG. Can hierarchical modeling improve our understanding of bovine abortion due to Neospora caninum infection?. Vet. Parasitol. 2017; 237: 77-82. doi: https://doi.org/f959f9 DOI: https://doi.org/10.1016/j.vetpar.2017.02.016
Li XJ. Sero-epidemiological investigation and analysis of neosporosis in dairy cattle of Jiyuan. J. Tradit. Chin. Vet. Med. 2015; 10:18-19.
Ribeiro CM, Soares IR, Mendes RG, de Santis-Bastos PA, Katagiri S, Zavilenski RB, Porto-de Abreu HF, Afreixo V. Meta-analysis of the prevalence and risk factors associated with bovine neosporosis. Trop. Anim. Health Prod. 2019; 51(7):1783-1800. doi: https://doi.org/pjbj DOI: https://doi.org/10.1007/s11250-019-01929-8
Rivera JEM, Hecker YP, Burucúa MM, Cirone KM, Cheuquepán FA, Fiorani F, Dorsch MA, Colque LA, Cantón GJ, Marin MS, Moore DP. Innate and humoral immune parameters at delivery in colostrum and calves from heifers experimentally infected with Neospora caninum. Mol. Immunol. 2021; 132:53-59. doi: https://doi.org/pjbk DOI: https://doi.org/10.1016/j.molimm.2021.01.016
Tang Z, Wang Z, Ma Z, Jin W, Lin S, Wang L, Min P, Li L, Zhao J, Jia L. Studies on experimental animals immunized with different antigenic vaccine combinations of Neospora caninum of cattle origin. Parasit Vectors. 2025; 18:49. doi: https://doi.org/pjbm DOI: https://doi.org/10.1186/s13071-025-06687-1
Williams DJL, Trees AJ. Protecting babies: vaccine strategies to prevent foetopathy in Neospora caninuminfected cattle. Parasite. Immunol. [Internet]. 2006; 28(3):61-67. doi: https://doi.org/frgfrj DOI: https://doi.org/10.1111/j.1365-3024.2005.00809.x
Dittrich RL, Regidor-Cerrillo J, Ortega-Mora LM, de Oliveira-Koch M, Busch APB, Gonçalves KA, Cruz AA. Isolation of Neospora caninum from kidney and brain of a bovine foetus and molecular characterisation in Brazil. Exp. Parasitol. [Internet]. 2018; 185:10-16. doi: https://doi.org/pjbp DOI: https://doi.org/10.1016/j.exppara.2018.01.004
Dubey JP, Schares G. Diagnosis of bovine neosporosis. Vet. Parasitol. [Internet]. 2006; 140(1-2):1-34. doi: https://doi.org/fkgpv2 DOI: https://doi.org/10.1016/j.vetpar.2006.03.035
García-Sánchez M, Jiménez-Pelayo L, Horcajo P, Collantes-Fernández E, Ortega-Mora LM, Regidor-Cerrillo J. Neospora caninum infection induces an isolate virulencedependent pro-inflammatory gene expression profile in bovine monocyte-derived macrophages. Parasite. Vectors. [Internet]. 2020; 13:1-20. doi: https://doi.org/pjbq DOI: https://doi.org/10.1186/s13071-020-04239-3
Jiménez-Pelayo L, García-Sánchez M, Regidor-Cerrillo J, Horcajo P, Collantes-Fernández E, Gómez-Bautista M, Hambruch N, Pfarrer C, Ortega-Mora LM. Differential susceptibility of bovine caruncular and trophoblast cell lines to infection with high and low virulence isolates of Neospora caninum. Parasite. Vectors. [Internet]. 2017; 10:1-13. doi: https://doi.org/pjbr DOI: https://doi.org/10.1186/s13071-017-2409-9
Hecker YP, Burucúa MM, Fiorani F, Maldonado-Rivera JE, Cirone KM, Dorsch MA, Cheuquepán FA, Campero LM, Cantón GJ, Marín MS, Ortega-Mora LM, Moore DP. Reactivation and Foetal Infection in Pregnant Heifers Infected with Neospora caninum Live Tachyzoites at Prepubertal Age. Vaccines. [Internet]. 2022; 10(8):1175. doi: https://doi.org/pjbs DOI: https://doi.org/10.3390/vaccines10081175
Goodswen SJ, Kennedy PJ, Ellis JT. A review of the infection, genetics, and evolution of Neospora caninum: from the past to the present. Infect. Gen. Evol. [Internet]. 2013; 13:133-150. doi: https://doi.org/f4htgf DOI: https://doi.org/10.1016/j.meegid.2012.08.012
Tramuta C, Lacerenza D, Zoppi S, Goria M, Dondo A, Ferroglio E, Patrizia N, Sergio R. Development of a set of multiplex standard polymerase chain reaction assays for the identification of infectious agents from aborted bovine clinical samples. J. Vet. Diagn. Invest. [Internet]. 2011; 23(4):657-664. doi: https://doi.org/dn9bmz DOI: https://doi.org/10.1177/1040638711407880
Rojo-Montejo S, Collantes-Fernández E, López-Pérez I, Risco-Castillo V, Prenafeta A, Ortega-Mora LM. Evaluation of the protection conferred by a naturally attenuated Neospora caninum isolate against congenital and cerebral neosporosis in mice. Vet. Res. [Internet]. 2012; 43:1-10. doi: https://doi.org/pjbv DOI: https://doi.org/10.1186/1297-9716-43-62
Marugán-Hernández V, Ortega-Mora LM, Aguado-Martínez A, Alvarez-García G. Genetic manipulation of Neospora caninum to express the bradyzoite-specific protein NcSAG4 in tachyzoites. Parasitology. [Internet]. 2011; 138(4):472-480. doi: https://doi.org/dd3v8x DOI: https://doi.org/10.1017/S0031182010001666
Gonçalves-Pereira KA, de Sousa RS, Varaschin MS, Busch-Becker APB, Gomes-Monteiro AL, Koch MO, Costa RC, Laskoski LM, Galindo CM, de Cristo TG, Fonseca FM, Locatelli-Dittrich R. Transplacental transmission of Neospora caninum to lambs in successive pregnancies of naturally infected sheep in Southern Brazil. Vet. Parasitol. Reg. Stud. Reports. [Internet]. 2021; 23:100537. doi: https://doi.org/pjbw DOI: https://doi.org/10.1016/j.vprsr.2021.100537
Abdelbaky HH, Shimoda N, Akthar I, Nakamura S, Hasan MH, Ushio N, Miyamoto A, Nishikawa Y. In vitro regulation of gene expression of pregnancy-associated proteins and cytokines in bovine endometrial epithelial cells and bovine trophoblastic cells by infection with Neospora caninum. Parasitol. Int. [Internet]. 2024; 101:102898. doi: https://doi.org/pjbx DOI: https://doi.org/10.1016/j.parint.2024.102898
Villa L, Gazzonis AL, Fumagalli E, Zanzani SA, Manfredi MT. The Utility of Serological Analysis for Neospora caninum Infection in Dairy Cattle Farms Management: Serological Investigation and Evaluation of the Effects on Reproductive and Productive Performances in Two Study Herds in Northern Italy. Animals. [Internet]. 2022; 12(6):786. doi: https://doi.org/pjbz DOI: https://doi.org/10.3390/ani12060786
Qian Y, Jiang Y, Hong H, Gao X, Liu W, Chen M, Jin Q, Jin Z, Li X, Wang X, Li J, Liu Q, Zhang X, Zhang N, Wei Z. Pathological characteristics and congenital immunological responses of pigeons-infected with Neospora caninum. Microb. Pathog. [Internet]. 2023; 182:106224. doi: https://doi.org/pjb2 DOI: https://doi.org/10.1016/j.micpath.2023.106224
Nayeri T, Shahabeddin S, Moosazadeh M, Daryani A. The Global Prevalence of Neospora caninum Infection in Sheep and Goats That Had an Abortion and Aborted Fetuses: A Systematic Review and Meta-Analysis. Front. Vet. Sci. [Internet]. 2022; 9:870904. doi: https://doi.org/pjb3 DOI: https://doi.org/10.3389/fvets.2022.870904
González-Warleta M, Castro-Hermida JA, Regidor-Cerrillo J, Benavides J, Álvarez-García G, Fuertes M, Ortega-Mora LM, Mercedes M. Neospora caninum infection as a cause of reproductive failure in a sheep flock. Vet. Res. [Internet]. 2014; 45(1):88. doi: https://doi.org/f6fp9m DOI: https://doi.org/10.1186/PREACCEPT-1141386470129662
Pita-Gondim LF, McAllister MM. Experimental Neospora caninum Infection in Pregnant Cattle: Different Outcomes Between Inoculation With Tachyzoites and Oocysts. Front. Vet. Sci. [Internet]. 2022; 9:911015. doi: https://doi.org/pjb4 DOI: https://doi.org/10.3389/fvets.2022.911015
Bezerra RA, Valencio BA, Ventura-Alvares FB, Alcântara ÉT, Sarmento WF, Bandeira-Melo RP, Mota RA, Azevedo SS, Gennari SM, Ribeiro-Vilela VL, Ferreira-Feitosa T. Dynamics of Neospora caninum transmission in naturally infected sheep under semiarid conditions. Small Rumin. Res. [Internet]. 2022; 217:106843. doi: https://doi.org/pjb5 DOI: https://doi.org/10.1016/j.smallrumres.2022.106843
Costa FTR, Nogueira DB, Oliveira MAG, Silva SS, Silva RF, Sarmento WF, Azevedo SS, Gennari SM, Pena HFJ, Brasil AWL, Vilela VLR, Feitosa TF. Vertical transmission of Toxoplasma gondii in naturally infected ewes in the semiarid region of Brazil. Comp. Immunol. Microbiol. Infect. Dis. [Internet]. 2021; 74:101595. doi: https://doi.org/pjb6 DOI: https://doi.org/10.1016/j.cimid.2020.101595
McAllister MM, McGuire AM, Jolley WR, Lindsay DS, Trees AJ, Stobart RH. Experimental neosporosis in pregnant ewes and their offspring. Vet. Pathol. [Internet]. 1996; 33(6):647-655. doi: https://doi.org/dctc48 DOI: https://doi.org/10.1177/030098589603300603
Feitosa TF, Ribeiro-Costa FT, Bezerra RA, Ventura-Álvares FB, Ferreira LC, Mota RA, Gennari SM, Pena HFJ, Azevedo SS, Ribeiro-Vilela VL. Vertical transmission and kinetic of antibodies anti-Neospora caninum in naturally infected lambs in the semiarid region of Brazil. Rev. Bras. Parasitol. Vet. [Internet]. 2021; 30(3):010621. doi: https://doi.org/pjb7 DOI: https://doi.org/10.1590/s1984-29612021073
González-Warleta M, Castro-Hermida JA, Regidor-Cerrillo J, Benavides J, Álvarez-García G, Fuertes M, Ortenga-Mora LM, Mezo M. Neospora caninum infection as a cause of reproductive failure in a sheep flock. Vet. Res. [Internet]. 2014; 45(1):1-9. doi: https://doi.org/f6fp9m
Masala G, Porcu R, Daga C, Denti S, Canu G, Patta C, Tola S. Detection of pathogens in ovine and caprine abortion samples from Sardinia, Italy, by PCR. J. Vet. Diagn. Invest. [Internet]. 2007; 19(1):96-98. doi: https://doi.org/bbc2sj DOI: https://doi.org/10.1177/104063870701900116
Benavides J, Collantes-Fernandez E, Ferre I, Perez V, Campero C, Mota R, Innes E, Ortega-Mora LM. Experimental ruminant models for bovine neosporosis: what is known and what is needed. Parasitology. [Internet]. 2014; 141(11):1471-1488. doi: https://doi.org/f6d357 DOI: https://doi.org/10.1017/S0031182014000638
Sánchez-Sánchez R, Ferre I, Re M, Regidor-Cerrillo J, Blanco-Murcia J, Ferrer LM, Navarro T, Pizarro-Díaz M, González-Huecas M, Tabanera E, Benavides J, OrtegaMora LM. Influence of dose and route of administration on the outcome of infection with the virulent Neospora caninum isolate Nc-Spain7 in pregnant sheep at midgestation. Vet. Res. [Internet]. 2018; 49:1-15. doi: https://doi.org/gdkjvc DOI: https://doi.org/10.1186/s13567-018-0539-5
Almería S, Serrano-Perez B, Darwich L, Domingo M, MurNovales R, Regidor-Cerrillo J, Cabezón O, Pérez-Maillo M, Lopez-Helguera I, Fernández-Aguilar X, Puig-Ribas M, Ortega-Mora LM, García-Ispierto I, Dubey JP, López-Gatius F. Foetal death in naive heifers inoculated with Neospora caninum isolate Nc-Spain7 at 110 days of pregnancy. Exp. Parasitol. [Internet]. 2016; 168:62-69. doi: https://doi.org/f8zxhc DOI: https://doi.org/10.1016/j.exppara.2016.06.009
Arranz-Solis D, Benavides J, Regidor-Cerrillo J, Fuertes M, Ferre I, Ferreras MC, Collantes-Fernández E, Hemphill A, Pérez V, Ortega-Mora LM. Influence of the gestational stage on the clinical course, lesional development and parasite distribution in experimental ovine neosporosis. Vet. Res. [Internet]. 2015; 46:19. doi: https://doi.org/f63f25 DOI: https://doi.org/10.1186/s13567-014-0139-y
Weston JF, Howe L, Collett MG, Pattison RS, Williamson NB, West DM, Pomroy WE, Syed-Hussain SS, Morris ST, Kenyon PR. Dose-titration challenge of young pregnant sheep with Neospora caninum tachyzoites. Vet. Parasitol. [Internet]. 2009; 164(1-2):183-191. doi: https://doi.org/bx9rc5 DOI: https://doi.org/10.1016/j.vetpar.2009.05.013
Nascimento-Porto WJ, Regidor-Cerrillo J, PeixotoKim PC, Benavides J, dos Santos-Silva AC, Horcajo P, da Fonseca-Oliveira AA, Ferre I, Mota RA, Ortega-Mora LM. Experimental caprine neosporosis: the influence of gestational stage on the outcome of infection. Vet. Res. [Internet]. 2016; 47:29. doi: https://doi.org/f79dv3 DOI: https://doi.org/10.1186/s13567-016-0312-6
Bień J, Moskwa B, Cabaj W. In vitro isolation and identification of the first Neospora caninum isolate from European bison (Bison bonasus bonasus L.). Vet. Parasitol. [Internet]. 2010; 173(3-4):200-205. doi: https://doi.org/b7q456 DOI: https://doi.org/10.1016/j.vetpar.2010.06.038
Costa RC, Pereira-Mesquita L, de Oliveira-Jr IM, Zannato DA, dos Santos-Mesquita LE, Biihrer DA, Salles-Gomes COM, Varaschin MS, Maiorka PC. The pathogenicity of two Neospora caninum goat strains in a BALB/c mouse model. Exp. Parasitol. [Internet]. 2019; 205:107736. doi: https://doi.org/pjcb DOI: https://doi.org/10.1016/j.exppara.2019.107736
González-Warleta M, Castro-Hermida JA, Regidor-Cerrillo J, Benavides J, Álvarez-García G, Fuertes M, Ortega-Mora LM, Mezo M. Neospora caninum infection as a cause of reproductive failure in a sheep flock. Vet. Res. [Internet]. 2014; 45:1-9. doi: https://doi.org/f6fp9m DOI: https://doi.org/10.1186/s13567-014-0088-5
Razmi G, Naseri Z. Molecular detection of Neospora caninum infection in ovine aborted foetuses in the Mashhad area, Iran. Ann. Parasitol. [Internet]. 2017; 63(4):5-47. doi: https://doi.org/pjcc
Yang N, Cui X, Qian W, Yu S, Liu Q. Survey of nine abortifacient infectious agents in aborted bovine fetuses from dairy farms in Beijing, China, by PCR. Acta. Vet. Hung. [Internet]. 2012; 60(1):83-92. doi: https://doi.org/pjcd DOI: https://doi.org/10.1556/avet.2012.007