Efectos y propiedades antimicrobianas del calostro liofilizado de diferentes especies sobre líneas celulares de cáncer de mama y páncreas
Resumen
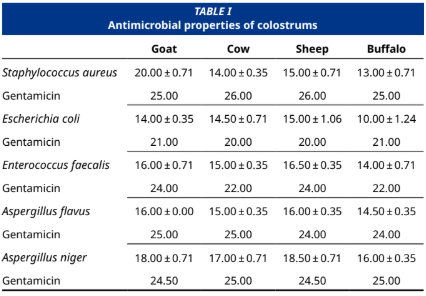
El calostro de mamíferos (también conocido como «oro líquido») es un alimento funcional de gran valor comercial y potencial futuro, ya que contiene altos niveles de nutrientes esenciales. En este estudio, obtuvimos calostro de ovejas Karayaka (Ovis aries), cabras Saanen (Capra aegagrus hircus), vacas rojas danesas (Bos taurus) y búfalos (Bubalus bubalis) y analizamos su efecto en las líneas celulares de cáncer de mama MCF–7 y de cáncer de páncreas AsPC–1 a diferentes dosis. Además, se determinó la actividad antimicrobiana potencial de estos calostros contra cinco bacterias/ mohos diferentes. la posible actividad antimicrobiana de estos calostros contra cinco bacterias y mohos diferentes. Para ello, se determinó la actividad antimicrobiana de los calostros liofilizados y desecados mediante el método de difusión en disco, y se evaluó la viabilidad celular y la citotoxicidad mediante la prueba MTT, así como los potenciales de migración mediante ensayos de cicatrización de heridas. Los resultados mostraron que estos calostros tenían actividades citotóxicas similares en las líneas celulares de cáncer AsPC–1 y MCF–7, con algunas diferencias menores. Además, en los ensayos de migración celular, la línea celular MCF–7 tratada con calostro de vaca mostró el mayor cierre de heridas en comparación con los demás calostros utilizados en el estudio. También descubrimos que los calostros de cabra y oveja tienen un mayor efecto antimicrobiano sobre Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, Aspergillus flavus y Aspergillus niger que los de vaca y búfala. El calostro de cabra tuvo el mayor efecto antimicrobiano sobre S. aureus, mientras que el calostro de búfala tuvo el menor efecto antimicrobiano sobre E. coli. En conclusión, los análisis de las actividades anticancerígenas y antimicrobianas del calostro entre especies arrojan luz sobre sus posibles beneficios para la salud y la producción de alimentos funcionales.
Descargas
Citas
El–Loly MM. Colostrum ingredients, its nutritional and health benefits–an overview. Clin. Nutr. Open Sci. [Internet]. 2022; 44:126-143. doi: https://doi.org/pg6f DOI: https://doi.org/10.1016/j.nutos.2022.07.001
Ayar A, Sıçramaz H, Çetin İ. The Effect of Bovine Colostrum on the Lactic Flora of Yogurt and Kefir. JSM Biotechnol. Bioeng. [Internet]. 2016 [cited 12 Dec. 2024]; 3(4):1063. Available in: https://goo.su/OHKA
Artym J, Zimecki M. Colostrum proteins in protection against therapy–induced injuries in cancer chemo–and radiotherapy: a comprehensive review. Biomedicines [Internet]. 2023; 11(1):114. doi: https://doi.org/pg6g DOI: https://doi.org/10.3390/biomedicines11010114
Silva EGDSO, Rangel AHDN, Mürmam L, Bezerra MF, Olıveıra JPFD. Bovine colostrum: benefits of its use in human food. Food Sci. Technol. [Internet]. 2019; 39(2):355-362. doi: https://doi.org/gn23x2 DOI: https://doi.org/10.1590/fst.14619
Anderson RC, Dalziel JE, Haggarty NW, Dunstan KE, Gopal PK, Roy NC. Processed bovine colostrum milk protein concentrate increases the epithelial barrier integrity of Caco-2 cell layers. J. Dairy Sci. [Internet]. 2019; 102(12):10772-10778. doi: https://doi.org/pg6h DOI: https://doi.org/10.3168/jds.2019-16951
Reddy RS, Ramachandra CT, Hiregoudar S, Nidoni U, Ram J, Kammar M. Influence of processing conditions on functional and reconstitution properties of milk powder made from Osmanabadi goat milk by spray drying. Small Rum. Res. [Internet]. 2014; 119(1–3):130–137. doi: https://doi.org/f545rg DOI: https://doi.org/10.1016/j.smallrumres.2014.01.013
Bagwe–Parab S, Yadav P, Kaur G, Tuli HS, Buttar HS. Therapeutic applications of human and bovine colostrum in the treatment of gastrointestinal diseases and distinctive cancer types: The current evidence. Front. Pharmacol. [Internet]. 2020; 11:01100. doi: https://doi.org/gn23xv DOI: https://doi.org/10.3389/fphar.2020.01100
Eker F, Akdaşçi E, Duman H, Yalçıntaş YM, Canbolat AA, Kalkan AE, Karev S, Šamec D. Antimicrobial properties of colostrum and milk. Antibiotics [Internet]. 2024; 13(3):251. doi: https://doi.org/pg6j DOI: https://doi.org/10.3390/antibiotics13030251
Giorgio D, Di Trana A, Claps S. Oligosaccharides, polyamines and sphingolipids in ruminant milk. Small Rumin. Res. [Internet]. 2018; 160:23-30. doi: https://doi.org/gdbgcx DOI: https://doi.org/10.1016/j.smallrumres.2018.01.006
Maldonado–Gomez MX, Lee H, Barile D, Lu M, Hutkins RW. Adherence inhibition of enteric pathogens to epithelial cells by bovine colostrum fractions. Intern. Dairy J. [Internet]. 2015; 40:24-32. doi: https://doi.org/pg6k DOI: https://doi.org/10.1016/j.idairyj.2014.08.014
Kowalczyk P, Kaczyńska K, Kleczkowska P, Bukowska–Ośko I, Kramkowski K, Sulejczak D. The lactoferrin phenomenon—a miracle molecule. Molecules [Internet]. 2022; 27(9):2941. doi: https://doi.org/pg6m DOI: https://doi.org/10.3390/molecules27092941
Maga EA, Desai PT, Weimer BC, Dao N, Kültz D, Murray JD. Consumption of lysozyme–rich milk can alter microbial fecal populations. App. Environ. Microbiol. [Internet]. 2012; 78(17):6153-6160. doi: https://doi.org/f369gd DOI: https://doi.org/10.1128/AEM.00956-12
Singh A, Duche RT, Wandhare AG, Sian JK, Singh BP, Sihag MK, Singh KS, Sangwan V, Talan S, Panwar H. Milk–derived antimicrobial peptides: overview, applications, and future perspectives. Probiotics Antimicrob. Prot. [Internet]. 2023; 15(1):44-62. doi: https://doi.org/pg6n DOI: https://doi.org/10.1007/s12602-022-10004-y
Diarra MS, Petitclerc D, Deschênes E, Lessard N, Grondin G, Talbot BG, Lacasse P. Lactoferrin against Staphylococcus aureus Mastitis: Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. [Internet]. 2003; 95(1-2):33-42. doi: https://doi.org/bb2mrd DOI: https://doi.org/10.1016/S0165-2427(03)00098-9
Rybarczyk J, Kieckens E, Vanrompay D, Cox E. In vitro and in vivo studies on the antimicrobial effect of lactoferrin against Escherichia coli O157: H7. Vet. Microbiol. [Internet]. 2017; 202:23-28. doi: https://doi.org/gbphqm DOI: https://doi.org/10.1016/j.vetmic.2016.05.010
Menadi S, Kucuk B, Cacan E. Promoter hypomethylation upregulates ANXA2 expression in pancreatic cancer and is associated with poor prognosis. Biochem. Genet. [Internet]. 2024;62:2721–2742. doi: https://doi.org/pg6p DOI: https://doi.org/10.1007/s10528-023-10577-5
Criscitiello C, Corti C. Breast cancer genetics: diagnostics and treatment. Genes [Internet]. 2022; 13(9):1593. doi: https://doi.org/pg6q DOI: https://doi.org/10.3390/genes13091593
Miranda C, Igrejas G, Poeta P. Bovine Colostrum: Human and Animal health benefits or route transmission of antibiotic resistance—One Health perspective. Antibiotics [Internet]. 2023; 12(7):1156. doi: https://doi.org/pg6r DOI: https://doi.org/10.3390/antibiotics12071156
Lotito D, Pacifico E, Matuozzo S, Musco N, Iommelli P, Zicarelli F, Tudisco R, Infascelli F, Lombardi P. Colostrum composition, characteristics and management for buffalo calves: A review. Vet. Sci. [Internet]. 2023; 10(5):358. doi: https://doi.org/pg6s DOI: https://doi.org/10.3390/vetsci10050358
Sharma A, Shandilya UK, Sodhi M, Mohanty AK, Jain P, Mukesh M. Evaluation of Milk colostrum derived Lactoferrin of Sahiwal (Bos indicus) and Karan fries (cross–bred) cows for its anti–cancerous potential. Int. J. Mol. Sci. [Internet]. 2019; 20(24):6318. doi: https://doi.org/pg6t DOI: https://doi.org/10.3390/ijms20246318
Amiri F, Moradian F, Rafiei A. Anticancer effect of lactoferrin on gastric cancer cell line AGS. Res. Mol. Med. [Internet]. 2015; 3(2):11-16. doi: https://doi.org/pg6w
Farziyan MA, Moradian F, Rafiei AR. Anticancer effect of bovine lactoferrin on human esophagus cancer cell line. Res. Mol. Med. [Internet]. 2016; 4(1):18-23. doi: https://doi.org/pg6x
Shahzad MM, Felder M, Ludwig K, Van Galder HR, Anderson ML, Kim J, Cook ME, Kapur AK, Patankar MS. Trans10, cis12 conjugated linoleic acid inhibits proliferation and migration of ovarian cancer cells by inducing ER stress, autophagy, and modulation of Src. PLoS One [Internet]. 2018; 13(1):e0189524. doi: https://doi.org/gctt7m DOI: https://doi.org/10.1371/journal.pone.0189524
Sugihara Y, Zuo X, Takata T, Jin S, Miyauti M, Isikado A, Imanaka H, Tatsuka M, Qi G, Shimamoto F. Inhibition of DMHDSSinduced colorectal cancer by liposomal bovine lactoferrin in rats. Oncol. Lett. [Internet]. 2017; 14(5):5688- 5694. doi: https://doi.org/gch2vd DOI: https://doi.org/10.3892/ol.2017.6976
Abu–Serie MM, El–Fakharany EM. Efficiency of novel nanocombinations of bovine milk proteins (lactoperoxidase and lactoferrin) for combating different human cancer cell lines. Sci. Rep. [Internet]. 2017; 7(1):16769, doi: https://doi.org/pg6z DOI: https://doi.org/10.1038/s41598-017-16962-6
Niezgoda N, Gliszczyńska A, Kempińska K, Wietrzyk J, Wawrzeńczyk C. Synthesis and evaluation of the cytotoxic activity of conjugated linoleic acid derivatives (esters, alcohols, and their acetates) toward cancer cell lines. Eur. J. Lipid Sci. Technol. [Internet]. 2017; 119(10):1600470. doi: https://doi.org/pg62 DOI: https://doi.org/10.1002/ejlt.201600470
Alsayed AR, Hasoun LZ, Khader HA, Basheti IA, Permana AD. Bovine Colostrum Treatment of Specific Cancer Types: Current Evidence and Future Opportunities. Molecules [Internet]. 2022; 27(24):8641. doi: https://doi.org/pg63 DOI: https://doi.org/10.3390/molecules27248641
Ebrahimabadi AH, Djafari–Bidgoli Z, Mazoochi A, Kashi FJ, Batooli H. Essential oils composition, antioxidant and antimicrobial activity of the leaves and flowers of Chaerophyllum macropodum Boiss. Food Control [Internet]. 2010; 21(8):1173- 1178. doi: https://doi.org/d6w3nn DOI: https://doi.org/10.1016/j.foodcont.2010.01.014
Oskay M, Aktaş K, Sarı D, Azeri C. Asphodelus aestivus (Liliaceae)’un antimikrobiyal etkisinin çukur ve disk diffüzyon yöntemiyle karşılaştırmalı olarak belirlenmesi [A comparative study of antimicrobial activity using well and disk diffusion method on Asphodelus aestivus (Liliaceae)]. Ekoloji. [Internet]. 2007 [cited 12 Dec. 2024]; 16(62):62-65. Turkish. Available in: https://goo.su/xkc3
Berkel C, Cacan E. In silico analysis of DYNLL1 expression in ovarian cancer chemoresistance. Cell Bio. Int. [Internet]. 2020; 44(8):1598-1605. doi: https://doi.org/gk58rp DOI: https://doi.org/10.1002/cbin.11352
Berkel C, Cacan E. Involvement of ATMIN–DYNLL1-MRN axis in the progression and aggressiveness of serous ovarian cancer. Biochem. Biophys. Res. Commun. [Internet]. 2021; 570:74–81. doi: https://doi.org/pg7v DOI: https://doi.org/10.1016/j.bbrc.2021.07.004
Cacan E, Ozmen ZC. Regulation of Fas in response to bortezomib and epirubicin in colorectal cancer cells. J. Chemother. [Internet]. 2020; 32(4):193–201. doi: https://doi.org/pg7t DOI: https://doi.org/10.1080/1120009X.2020.1740389
Gülmez Y, Aydın A, Can İ, Tekin Ş, Cacan E. Cellular toxicity and biological activities of honey bee (Apis mellifera L.) venom. Marmara Pharm. J. [Internet]. 2017; 21(2):251-260. doi: https://doi.org/pg7w DOI: https://doi.org/10.12991/marupj.300329
Ariffin SMZ, Hasmadi N, Syawari NM, Sukiman MZ, Ariffin MFT, Hian CM, Ghazali MF. Prevalence and antibiotic susceptibility pattern of Staphylococcus aureus, Streptococcus agalactiae, and Escherichia coli in dairy goats with clinical and subclinical mastitis. J. Anim. Health Prod. [Internet]. 2019; 7(1):32-37. doi: https://doi.org/pg7x DOI: https://doi.org/10.17582/journal.jahp/2019/7.1.32.37
Mansor R, Diauudin NS, Syed–Hussain SS, Khalid SF. Antibiotic susceptibility of Staphylococcus aureus and Escherichia coli isolated from dairy goats in selected farms in Selangor, Malaysia. J. Vet. Malaysia. [Internet]. 2019; 31(1):12-16. doı: https://doi.org/pg7z DOI: https://doi.org/10.71118/586246
Bantaw K, Sah SN, Subba Limbu D, Subba P, Ghimire A. Antibiotic resistance patterns of Staphylococcus aureus, Escherichia coli, Salmonella, Shigella and Vibrio isolated from chicken, pork, buffalo and goat meat in eastern Nepal. BMC Res. Notes [Internet]. 2019; 12(1):766. doi: https://doi.org/pg72 DOI: https://doi.org/10.1186/s13104-019-4798-7
Rana EA, Fazal MA, Alim MA. Frequently used therapeutic antimicrobials and their resistance patterns on Staphylococcus aureus and Escherichia coli in mastitis affected lactating cows. Int. J. Vet. Sci. Med. [Internet]. 2022; 10(1):1-10. doi: https://doi.org/pg73 DOI: https://doi.org/10.1080/23144599.2022.2038494
Shahidi F, Roshanak S, Javadmanesh A, Tabatabaei Yazdi F, Pirkhezranian Z, Azghandi M. Evaluation of antimicrobial properties of bovine lactoferrin against foodborne pathogenic microorganisms in planktonic and biofilm forms (in vitro). J. Consum. Prot. Food S. [Internet]. 2020; 15:277-283. doi: https://doi.org/gn99n5 DOI: https://doi.org/10.1007/s00003-020-01280-3
Bursalioglu EO. Effect of cow colostrum, mare milk, and human milk on the viability of lung healthy and cancer cell lines. Iran Red. Crescent Med. J. [Internet]. 2021; 23(5):e409. doi: https://doi.org/pg74
Akca C, Vatan O, Yilmaz D, Huriyet H, Cinkilic N, Cavas T. In vitro cytotoxic and genotoxic ef fects of donkey milk on lung cancer and normal cells lines. Czech J. Food Sci. [Internet]. 2019; 37(1):29-35. doi: https://doi.org/pg75 DOI: https://doi.org/10.17221/221/2018-CJFS
Balagayathri R, Uma C, Sıvagurunathan P, Rao VD, Suman P. Assessment of In–vitro Antimicrobial, Antioxidant and anticancer activity of bioactive peptides of the Goat Colostrum. Int. J. Pharma. Res. [Internet]. 2021; 13(3):1694-1708. doi: https://doi.org/pg8c DOI: https://doi.org/10.31838/ijpr/2021.13.03.234
Tikhonov S, Chernukha I, Dunchenko N. Comparative evaluation antimicrobial and antitumor activities of natural colostrum peptide and its synthesized analogue. Food Sci. App. Biotechnol. [Internet]. 2024; 7(2):333-343. doi: https://doi.org/pg8d DOI: https://doi.org/10.30721/fsab2024.v7.i2.283
Khan MZ, Xiao J, Ma Y, Ma J, Liu S, Khan A, Khan JM, Cao Z. Research development on anti–microbial and antioxidant properties of camel milk and its role as an anti–cancer and anti–hepatitis agent. Antioxidants. [Internet]. 2021; 10(5):788. doi: https://doi.org/k7tt DOI: https://doi.org/10.3390/antiox10050788
Bielecka M, Cichosz G, Czeczot H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates–A review. Int. Dairy J. [Internet]. 2022; 127:105208. doi: https://doi.org/pg8f DOI: https://doi.org/10.1016/j.idairyj.2021.105208
Agarwal P, Gupta R. A review on anticancer property of colostrum. Res. Rev. J. Med. Health Sci. 2016 [cited 12 Dec. 2024]; 5(4):1-9. Available in: https://goo.su/xwjU
Kim H, Kim DE, Han G, Lim NR, Kim EH, Jang Y, Cho H, Jang H, Kim KH, Kim SH, Yang Y. Harnessing the natural healing power of colostrum: bovine milk–derived extracellular vesicles from colostrum facilitating the transition from inflammation to tissue regeneration for accelerating cutaneous wound healing. Adv. Healthc. Mater. [Internet]. 2022; 11(6):2102027. doi: https://doi.org/pg8g DOI: https://doi.org/10.1002/adhm.202102027
Kelley NS, Hubbard NE, Erickson KL. Conjugated linoleic acid isomers and cancer. J. Nutr. [Internet]. 2007; 137(12):2599-2607. doi: https://doi.org/gfvmts DOI: https://doi.org/10.1093/jn/137.12.2599
Samuel M, Sanwlani R, Pathan M, Anand S, Johnston EL, Ang CS, Liaskos MK, Mathivanan S. Isolation and Characterization of Cow–, Buffalo–, Sheep–and Goat–Milk–Derived Extracellular Vesicles. Cells [Internet]. 2023; 12(20):2491. doi: https://doi.org/pg8j DOI: https://doi.org/10.3390/cells12202491
Mehra R, Sangwan K, Garhwal R. Composition and Therapeutic Applications of Goat Milk and Colostrum. J. Dairy Sci. Technol. [Internet]. 2021 [cited 12 Dec. 2024]; 10(2):1-7. Available in: https://goo.su/JmGZEM
Kim Y, Kim MJ, Han KS, Imm JY, Oh S, Kim SH. Anticancer activity of lactoferrin isolated from caprine colostrum on human cancer cell lines. Int. J. Dairy Technol. [Internet]. 2009; 62(2):277-281. doi: https://doi.org/bwjd5w DOI: https://doi.org/10.1111/j.1471-0307.2009.00466.x