Perfil de ácidos grasos en gametos de trucha de arroyo (Salvelinus fontinalis) cultivada en época de desove
Resumen
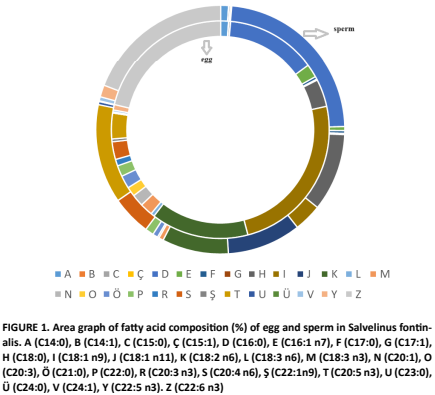
La fertilización y posterior desarrollo de embriones y/o larvas está relacionada con la calidad de los gametos y su composición en ácidos grasos (AG), siendo este uno de los factores específicos que afectan la calidad del óvulo o del esperma. Por lo tanto, evaluamos el perfil de AG de los gametos (óvulos y espermatozoides) en trucha de arroyo (Salvelinus fontinalis) en cultivo. Los AG se detectaron y midieron mediante cromatografía de gases. Nuestros resultados indicaron que se detectaton un total de 26 y 22 AG en óvulos y espermatozoides, respectivamente. Los niveles de AGPI se determinaron en cantidades casi idénticas tanto en óvulos (47,57%) como en espermatozoides (47,53%). DHA (ácido docosahexaenoico), LA (ácido linoleico), EPA (ácido eicosapentaenoico) y ARA (ácido araquidónico) fueron los PUFA (Ácido Graso Poliinsaturado) predominantes para óvulos y espermatozoides. Curiosamente, el ácido vaccénico (C18:1 n-11) (24,07%) fue el principal AG en el huevo. En conclusión, el análisis de los ácidos grasos en los gametos de los reproductores aporta información valiosa sobre los requisitos dietéticos necesarios para programas eficaces de cría en acuicultura.
Descargas
Citas
Pereira DM, Valentão P, Teixeira N, Andrade PB. Amino acids, fatty acids and sterols profile of some marine organisms from Portuguese waters. Food Chem. [Internet]. 2013; 141(3):2412-2417. doı: https://doi.org/pf6p DOI: https://doi.org/10.1016/j.foodchem.2013.04.120
Zhang X, Ning X, He X, Sun X, Yu X, Cheng Y, Yu RQ, Wu Y. Fatty acid composition analyses of commercially important fish species from the Pearl River Estuary, China. PLoS One. [Internet]. 2020; 15(1):e0228276 doı: https://doi.org/gj3c4x DOI: https://doi.org/10.1371/journal.pone.0228276
Aras NM, Haliloğlu Hİ, Atamanalp M. Balıklarda yağ asitlerinin önemi. Atatürk Univ. Ziraat Fak. Derg. [Internet]. 2002 [cited dd/mm/año]; 33(3):331-335. Available in: https://goo.su/re5kI
Bayır A, Sirkecioğlu AN, Aras NM, Aksakal E, Haliloğlu Hİ, Bayır M. Fatty acids of neutral and phospholipids of three endangered trout: Salmo trutta caspius Kessler, Salmo trutta labrax Pallas and Salmo trutta macrostigma Dumeril. Food Chem. [Internet]. 2010; 119(3):1050-1056. doi: https://doi.org/fbm28b DOI: https://doi.org/10.1016/j.foodchem.2009.07.064
Taşbozan O, Gökçe MA. Fatty acids in fish. In Catala A, editor. Fatty Acids. Argentina: InTech; [Internet]. 2017; 1:143-159. doi: https://doi.org/gk928j DOI: https://doi.org/10.5772/68048
Özçiçek E, Can E, Yılmaz Ö. Comparison of nutrient contents of wild and farmed rainbow trout (Oncorhynchus mykiss, Walbaum 1792) from Keban Dam Lake in Eastern Anatolia region of Turkey. Aquac. Res. [Internet]. 2022; 53:2457-2463. doi: https://doi.org/pf6t DOI: https://doi.org/10.1111/are.15763
Özçiçek E, Can E, Yılmaz Ö. Yetiştiriciliği yapılan ve doğadan avlanan gökkuşağı alabalığının (Oncorhynchus mykiss, Walbaum 1792) karaciğer dokusu besin düzeylerinin karşılaştırılması. Menba Kastamonu Univ. Su Ürünleri Fak. Derg. 2022 [cited Oct. 24 2024]; 8(2):94-104. Available in: https://goo.su/riJi
Bobe J, Labbé C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. [Internet]. 2010; 165(3):535-548. doi: https://doi.org/fm5pcd DOI: https://doi.org/10.1016/j.ygcen.2009.02.011
Ozaki Y, Koga H, Takahashi T, Adachi S, Yamauchi K. Lipid content and fatty acid composition of muscle, liver, ovary and eggs of captive-reared and wild silver Japanese eel (Anguilla japonica) during artificial maturation. Fish Sci. [Internet]. 2008; 74(2):362-371. doi: https://doi.org/c42f49 DOI: https://doi.org/10.1111/j.1444-2906.2008.01525.x
Diaz R, Torres MA, Bravo S, Sanchez R, Sepulveda N. Determination of fatty acid profile in ram spermatozoa and seminal plasma. Andrologia. [Internet]. 2015; 48(6):723-726. doi: https://doi.org/pf6q DOI: https://doi.org/10.1111/and.12506
Yuan C, Wang J, Lu W. Regulation of semen quality by fatty acids in diets, extender, and semen. Front Vet. Sci. [Internet]. 2023; 10:1119153. doı: https://doi.org/pf64 DOI: https://doi.org/10.3389/fvets.2023.1119153
Collodel G, Moretti E, Longini M, Pascarelli NA, Signorini C. Increased F2-isoprostane levels in semen and immunolocalization of the 8-iso prostaglandin F2α in spermatozoa from infertile patients with varicocele. Oxid. Med. Cell. Longev. [Internet]. 2018; 2018:7508014. doı: https://doi.org/gdfgtm DOI: https://doi.org/10.1155/2018/7508014
Collodel G, Castellini C, Lee JC, Signorini C. Relevance of fatty acids to sperm maturation and quality. Oxid. Med. Cell. Longev. [Internet]. 2020; 2020:7038124. doı: https://doi.org/pf9c DOI: https://doi.org/10.1155/2020/7038124
Dupont-Cyr BA, Le François NR, Christen F, Desrosiers V, Savoie A, Vandenberg GW, Dufresne F, Blier PU. Linseed oil as a substitute for fish oil in the diet of Arctic charr (Salvelinus alpinus), brook charr (S. fontinalis) and their reciprocal hybrids. Aquac. Rep. [Internet]. 2022; 22:100949. doı: https://doi.org/pf9d DOI: https://doi.org/10.1016/j.aqrep.2021.100949
Okumuş İ, Başçınar N. Studies on aquaculture potential of brook trout (Salvelinus fontinalis). Lessons From The Past to Optimise the Future, European Aquaculture Society, Trondheim, Norway; 5 - 08 August 2005. pp.346-347.
Atasaral Şahin Ş, Başçınar N, Kocabaş M, Tufan B, Köse S, Okumuş İ. Evaluation of meat yield, proximate composition and fatty acid profile of cultured brook trout (Salvelinus fontinalis Mitchill, 1814) and Black Sea trout (Salmo trutta labrax Pallas, 1811) in comparison with their hybrid. Turk. J. Fish. Aquat. Sci. [Internet]. 2011; 11(2):261-271. doi: https://doi.org/bw3737
Atchison GJ. Fatty acid levels in developing brook trout (Salvelinus fontinalis) eggs and fry. J. Fish. Board Can. [Internet]. 1975; 32(12):2513-2515. doı: https://doi.org/b2s5bb DOI: https://doi.org/10.1139/f75-289
Guillou A, Soucy P, Khalil M, Adambounou L. Effects of dietary vegetable and marine lipid on growth, muscle fatty acid composition and organoleptic quality of flesh of brook charr (Salvelinus fontinalis). Aquaculture. [Internet]. 1995; 136(3-4):351-362. doı: https://doi.org/bq3rc5 DOI: https://doi.org/10.1016/0044-8486(95)00053-4
Kleinová J, Brabec T, Mareš J. The spectrum of fatty acids in lipids of Salvelinus fontinalis in relation to the origin, feed and breeding density. Mendelnet. [Internet]. 2013 [cited 12 December 2024]; 216-220. Available in: https://goo.su/hQVlN
Zajic T, Mraz J, Sampels S, Pickova, J. Finishing feeding strategy as an instrument for modification of fatty acid composition of brook char (Salvelinus fontinalis). Aquac. Int. [Internet]. 2016; 24:1641-1656. doı: https://doi.org/f9fdj9 DOI: https://doi.org/10.1007/s10499-016-0067-0
Gladyshev MI, Makhrov AA, Baydarov IV, Safonova SS, Golod VM, Alekseyev SS, Glushchenko LA, Rudchenko AE, Karpov VA, Sushchik NN. Fatty acid composition and contents of fish of genus Salvelinus from natural ecosystems and aquaculture. Biomolecules. [Internet]. 2022; 12(1):144. doı: https://doi.org/gpd7gn DOI: https://doi.org/10.3390/biom12010144
Özyılmaz A, Ocak K, Demirci S. Divergences of biochemical features of three reared trouts; brook trout (Salvelinus fontinalis, Mitchill 1814), rainbow trout (Oncorhynchus mykiss Walbaum, 1972), and Black Sea trout (Salmo trutta labrax Pallas 1811). J. Agric. Nat. [Internet]. 2023; 26(1):192-200. doı: https://doi.org/pf9g DOI: https://doi.org/10.18016/ksutarimdoga.vi.1038290
Hara AR, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem. [Internet]. 1978; 90(1):420-426. doı: https://doi.org/b3c4h8 DOI: https://doi.org/10.1016/0003-2697(78)90046-5
Christie WW. Gas Chromatography and Lipids. The Oil Press, Glaskow. 1992; 302 p.
Labbé C, Crowe LM, Crowe JH. Stability of the lipid component of trout sperm plasma membrane during freeze–thawing. Cryobiology. [Internet]. 1997; 34(2):176-182. doı: https://doi.org/c7d44r DOI: https://doi.org/10.1006/cryo.1996.1996
Pustowka C, McNiven MA, Richardson GF, Lall SP. Source of dietary lipid affects sperm plasma membrane integrity and fertility in rainbow trout (Oncorhynchus mykiss, Walbaum) after cryopreservation. Aquac. Res. [Internet]. 2000; 31:297-305. doı: https://doi.org/fwwkcd DOI: https://doi.org/10.1046/j.1365-2109.2000.00416.x
Tocher DR. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. [Internet]. 2003; 11(2):107-184. doı: https://doi.org/cxj9zx DOI: https://doi.org/10.1080/713610925
Ramos-Júdez S, Estévez A, González-López WÁ, Duncan N. Lipid and fatty acid composition of muscle, liver, ovary, and peritoneal fat in wild flathead grey mullet (Mugil cephalus) according to ovarian development. Theriogenology. [Internet]. 2023; 198(1):317-326. doı: https://doi.org/pf9h DOI: https://doi.org/10.1016/j.theriogenology.2022.12.046
Aslan SS, Guven KC, Gezgin T, Alpaslan M, Tekinay A. Comparison of fatty acid contents of wild and cultured rainbow trout (Oncorhynchus mykiss) in Turkey. Fish. Sci. [Internet]. 2007; 73:1195-1198. doı: https://doi.org/dw9jpg DOI: https://doi.org/10.1111/j.1444-2906.2007.01452.x
Lahnsteiner F, Mansour N, McNiven MA, Richardson GF. Fatty acids of rainbow trout (Oncorhynchus mykiss) semen: composition and effects on sperm functionality. Aquaculture. [Internet]. 2009; 298(1-2):118-124. doı: https://doi.org/dmrhsw DOI: https://doi.org/10.1016/j.aquaculture.2009.08.034
Ashton HJ, Farkvam DO, March BE. Fatty acid composition of lipids in the eggs and alevins from wild and cultured chinook salmon (Oncorhynchus tshawytscha). Can. J. Fish. Aquat. Sci. [Internet]. 1993; 50(3):648-655. doı: https://doi.org/b2sf4q DOI: https://doi.org/10.1139/f93-074
Harliolu AG. Comparative study of fatty acid composition of gametes, embryos and larvae of rainbow trout, Oncorhynchus mykiss. Pak. J. Zool. [Internet]. 2017; 49(5):1803-1808. doi: https://doi.org/pgdc DOI: https://doi.org/10.17582/journal.pjz/2017.49.5.1803.1808
Güler GO, Zengin G, Çakmak YS, Aktumsek A. Comparison of fatty acid compositions and ω3/ω6 ratios of wild brown trout and cultured rainbow trout. Turk. J. Fish. Aquat. Sci. [Internet]. 2017; 17:1179-1187. doı: https://doi.org/pgdd
Çankırılıgil EC, Berik N. Chemical composition of the Black Sea trout (Salmo labrax Pallas 1814): A comparative study. Aquat. Res. [Internet]. 2020; 3(4):208-219. doı: https://doi.org/pgdf DOI: https://doi.org/10.3153/AR20019
Turgay Ö. Seasonal variation of fatty acid composition of trout (Salmo opimus). Batman Univ. Yaşam Bilim. Derg. 2020 [cited Sept. 13 2024]; 10(1):1-10. Available in: https://goo.su/3XdabvK
Baki B, Ozturk DK, Tomgisi S. Comparative analysis of egg biochemical composition and egg productivity rainbow trout (Oncorhynchus mykiss Walbaum, 1792) in different stations in Turkey. Aquac. Stud. [Internet]. 2021; 21(3):117-127. doi: https://doi.org/pgdg DOI: https://doi.org/10.4194/2618-6381-v21_3_04
Molversmyr E, Devle HM, Naess-Andresen CF, Ekeberg D. Identification and quantification of lipids in wild and farmed Atlantic salmon (Salmo salar), and salmon feed by GC-MS. Food Sci. Nutr. [Internet]. 2022; 10(9):3117-3127. doı: https://doi.org/pgdh DOI: https://doi.org/10.1002/fsn3.2911
Özgür ME, Erdogan S, Aydemir S, Yumusakbas H. Evaluation of relationship between sperm cell velocities and fatty acids contents of semen seminal fluid in the two trout fish species. Pak. J. Zool. [Internet]. 2023; 55(2):863-870. doı: https://doi.org/pgdj DOI: https://doi.org/10.17582/journal.pjz/20211112131153
Pérez MJ, Rodríguez C, Cejas JR, Martín MV, Jerez S, Lorenzo A. Lipid and fatty acid content in wild white seabream (Diplodus sargus) broodstock at different stages of the reproductive cycle. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. [Internet]. 2007; 146(2):187-196. doı: https://doi.org/d2zcjc DOI: https://doi.org/10.1016/j.cbpb.2006.10.097
Silversand C, Norberg B, Haux C. Fatty-acid composition of ovulated eggs from wild and cultured turbot (Scophthalmus maximus) in relation to yolk and oil globule lipids. Mar. Biol. [Internet]. 1996; 125:269-278. doı: https://doi.org/fthxq4 DOI: https://doi.org/10.1007/BF00346307
Hosseini SV, Abedian-Kenari A, Regenstein JM, Rezaei M, Nazari RM, Moghaddasi M, Kaboli SA, Grant AAM. Effects of alternative dietary lipid sources on growth performance and fatty acid composition of beluga sturgeon, Huso huso, juveniles. J. World Aquac. Soc. [Internet]. 2010; 41(4):471-489. doı: https://doi.org/cqbj83 DOI: https://doi.org/10.1111/j.1749-7345.2010.00389.x
Ovissipour M, Rasco B. Fatty acid and amino acid profiles of domestic and wild beluga (Huso huso) roe and impact on fertilization ratio. J. Aquac. Res. Dev. [Internet]. 2011; 2(3):113. doı: https://doi.org/cq7zg8 DOI: https://doi.org/10.4172/2155-9546.1000113
Czesny S, Dabrowski K. The effect of egg fatty acid concentrations on embryo viability in wild and domesticated walleye (Stizostedion vitreum). Aquat. Living Resour. [Internet]. 1998; 11(6):371-378. doı: https://doi.org/bnd58s DOI: https://doi.org/10.1016/S0990-7440(99)80002-3
Czesny S, Dabrowski K, Christensen JE, VanEenennaam JP, Doroshov SI. Discrimination of wild and domestic origin of sturgeon ova based on lipids and fatty acid analysis. Aquaculture. [Internet]. 2000; 189(1-2):145-153. doı: https://doi.org/fqpsd2 DOI: https://doi.org/10.1016/S0044-8486(00)00364-1
Gallagher ML, Paramore L, Alves D, Rulifson RA. Comparison of phospholipid and fatty acid composition of wild and cultured striped bass eggs. J. Fish Biol. [Internet]. 1998; 52(6):1218-1228. doı: https://doi.org/brm8kj DOI: https://doi.org/10.1111/j.1095-8649.1998.tb00967.x
Mansour N, Lahnsteiner F, McNiven MA, Richardson GF, Pelletier CS. Relationship between fertility and fatty acid profile of sperm and eggs in Arctic char, Salvelinus alpinus. Aquaculture. [Internet]. 2011; 318(3-4):371-378. doı: https://doi.org/fbvj4n DOI: https://doi.org/10.1016/j.aquaculture.2011.05.023