Distribución de cuatro proteínas homeobox en los compartimentos gástricos bovinos durante el período fetal
Resumen
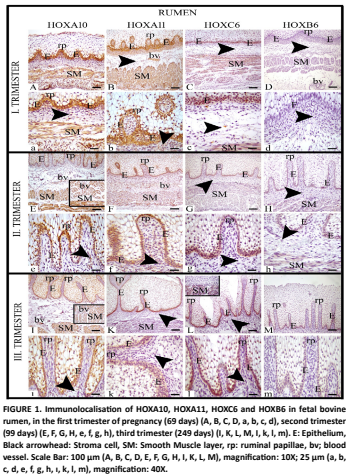
Las proteínas HOX/Hox, tiene funciones importantes en la determinación de la identidad celular durante el desarrollo embrionario, la diferenciación y la morfogénesis de las células madre embrionarias, así como en la formación y desarrollo del tracto gastrointestinal de los mamíferos. Con base en estos datos, este estudio fue diseñado para determinar la localización inmunohistoquímica y los niveles de expresión de HOXA10, HOXA11, HOXC6 y HOXB6, que son subunidades de las proteínas homeobox, en el rumen, retículo, omaso y abomaso durante el desarrollo fetal. Para ello, los fetos obtenidos de mataderos privados se agruparon según sus medidas de longitud cráneo-anca (LCC) y el trimestre gestacional al que pertenecían, de la siguiente manera: primer trimestre (69-89 días de edad/10 fetos), segundo trimestre (99-178 días/10 fetos) y tercer trimestre (188-269 días/10 fetos). Las muestras de tejido gástrico tomadas de cada grupo se sometieron a procesamiento histológico de rutina y tinción inmunohistoquímica. Como resultado de la tinción, se identificaron las proteínas HOXA10, HOXA11 y HOXC6; Se determinó que se expresaban con intensidades variables en el rumen, retículo, omaso y abomaso, y especialmente esta intensidad de expresión fue más fuerte en las células epiteliales y de la capa de músculo liso. Mientras que, la expresion de HOXB6 en el rumen fue casi inexistente en las células epiteliales a partir del segundo y tercer trimestre del gestacion, la inmunorreacción resultó negativa en todas las secciones restantes del estómago. Como resultado de estos hallazgos; Sugirió que algunas proteínas homeobox pueden tener una importancia crítica para el desarrollo, la morfogénesis y la histogénesis de los segmentos del estómago bovino fetal y, por lo tanto, contribuir al rendimiento y la productividad del ganado durante toda la vida, especialmente en términos de productividad de carne y leche.
Descargas
Citas
Jones KR, John RE, Sundaram V. Morpho-histological studies of the gastrointestinal tract of the orange-rumped agouti (Dasyprocta leporina Linnaeus, 1758), with special reference to morphometry and histometry. Animals (Basels). [Internet]. 2022; 12(19):2493. doi: https://doi.org/g6fz3h DOI: https://doi.org/10.3390/ani12192493
Hassan MAS, Karslı MA. The effects of some feed additives in nutrition of ruminant animals. IJVAR. [Internet]. 2022; 5(2):107-112. doi: https://doi.org/pcsf
Scala G, Corona M, Maruccio L. Structural, histochemical and immunocytochemical study of the forestomach mucosa in domestic ruminants. Anat. Histol. Embryol. [Internet]. 2011; 40(1):47-54. doi: https://doi.org/dpdxkt DOI: https://doi.org/10.1111/j.1439-0264.2010.01037.x
Swan GE, Groenwald HB. Morphological changes associated with the development of the ruminoreticulum in growing lambs fed different rations. Onderstepoort J.Vet. Res. [Internet]. 2000; 67(2):105114. PMID: 11028746. Available in: https://n9.cl/q3bxe2
Ozguden-Akkoç CG. Sindirim Sistemi. In: Kumas Kulualp, M., Tarakçi Gençer, B editor. Veteriner Histoloji Evcil Memeliler ve Kuslar. Malatya: Medipres. 2023; p. 236240.
Soni T, Goswami H, Panchal KM. Prenatal development of fore-stomach in small ruminants. Adv. Life Sci. [Internet]. 2016[cited Nov 6, 2024]; 5(22):10209-10215. Available in: https://goo.su/lu3ko DOI: https://doi.org/10.5455/jasa.20160409121545
Maclean JA, Chen MA, Wayne CM, Bruce SR, RaoM, Meistrich ML, Macleod C, Wilkinson MF. Rhox: a new homeobox gene cluster. Cell. [Internet]. 2005; 120(3):369-382. doi: https://doi.org/b9w5v9 DOI: https://doi.org/10.1016/j.cell.2004.12.022
Draftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr. Rev. [Internet]. 2006; 27(4):331-355. doi: https://doi.org/dtdnxf DOI: https://doi.org/10.1210/er.2005-0018
Bhatlekar S, Fields JZ, Boman BM. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. [Internet]. 2018; 1:1-15. doi: https://doi.org/gd44t9 DOI: https://doi.org/10.1155/2018/3569493
Deb P. Epigenetic mechanism of regulation of hox genes and neurotransmitters via hormones and lncrna. [doctoral thesis on the Internet]. The University of Texas at Arlington; 2017[cited 5 Sep 2024]; 175 p. Avialable in: https://goo.su/lx5K
Zacchetti G, Duboule D, Zakany J. Hox gene function in vertebrate gut morphogenesis: the case of the caecum. Development. [Internet]. 2007; 134(22):3967-3973. doi: https://doi.org/dffkfq DOI: https://doi.org/10.1242/dev.010991
Topaloglu U, Ketani MA. The distribution of some homeobox proteins in the bovine placenta duringgestation. Theriogenology. [Internet]. 2021; 166:71-82. doi: https://doi.org/pcsg DOI: https://doi.org/10.1016/j.theriogenology.2021.02.015
Du H, Taylor HS. The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med. [Internet]. 2016; 6(1):a023002. doi: https://doi.org/gsz86z DOI: https://doi.org/10.1101/cshperspect.a023002
Cui Y, Gao D, Linghu E, Zhan Q, Chen R, Brock MV, Herman JG, Guo M. Epigenetic changes and functional study of HOXA11 in human gastric cancer. Epigenomics.[Internet]. 2015; 7(2):201-213. doi: https://doi.org/f7ksg3 DOI: https://doi.org/10.2217/epi.14.92
Song C, Han Y, Luo H, Qin Z, Chen Z, Liu Y, Lu S, Sun H, Zhou C. HOXA10 induces BCL2 expression, inhibits apoptosis, and promotes cell proliferation in gastric cancer. Cancer Med. [Internet]. 2019; 8(12):5651-5661. doi: https://doi.org/pcsh DOI: https://doi.org/10.1002/cam4.2440
Topaloglu U, Sagsöz H, Akbalik ME, Ketani MA, Güney Saruhan B, Aydin N. The expression profile of some homeobox proteins in the bovine liver during prenatal development. Kafkas Univ. Vet. Fak. Derg. [Internet]. 2022; 28(5): 543-552. doi: https://doi.org/pcsj
Riz I, Lee HJ, Baxter KK, Behnam R, Hawley TS, Hawley RG. Transcriptional activation by TLX1/HOX11 involves Gro/TLE corepressors. Biochem. Biophys. Res. Commun. Internet]. 2009; 380(2):361-365. doi: https://doi.org/fp5gxk DOI: https://doi.org/10.1016/j.bbrc.2009.01.099
Bodey B, Bodey BJR, Siegel SE, Luck JV, Kaiser HE. Homeobox B3, B4, and C6 gene product expression in osteosarcomas as detected by immunocytochemistry. Anticancer Res. [Internet]. 2000; 20(4):2717-2721. PMID: 10953349. Available in: https://n9.cl/y991b
Lin J, He J, He X, Wang L, Xue M, Zhuo W, Si J, Wang K, Chen S. HOXC6 functions as an oncogene and isoform HOXC6-2 may play the primary role in gastric carcinogenesis. Dig. Dis. Sci. [Internet]. 2020; 70(3):2896-2906. doi: https://doi.org/pcsk DOI: https://doi.org/10.1007/s10620-019-06013-7
Zhang Q, Jin XS, Yang ZY, Wei M, Lui BY, Gu QL. Upregulated Hoxc6 expression is associated with poor survival in gastric cancer patients. Neoplasma. [Internet]. 2013; 60(4):439-445. doi: https://doi.org/f46mrh DOI: https://doi.org/10.4149/neo_2013_057
Duverger O, Morasso MI. Role of homeobox genes in the patterning, specification, and differentiation of ectodermal appendages in mammals. J. Cell. Physiol.[Internet]. 2008; 216(2):337-346. doi: https://doi.org/c34nw6 DOI: https://doi.org/10.1002/jcp.21491
Parker HJ, Krumlauf R. A Hox gene regulatory network for hindbrain segmentation. Curr. Top. Dev. Biol. [Internet]. 2020; 139:169-203. doi: https://doi.org/pcsm DOI: https://doi.org/10.1016/bs.ctdb.2020.03.001
Xu B, Geerts D, Bu Z, Ai J, Jin L, Li Y, Zhang H, Zhu G.Regulation of endometrial receptivity by the highly expressed HOXA9, HOXA11 and HOXD10 HOX-class homeobox genes. Hum. Reprod. [Internet]. 2014; 29(4):781-790. doi: https://doi.org/pcsn DOI: https://doi.org/10.1093/humrep/deu004
Mishra A, Modi D. Role of HOXA10 in pathologies of the endometrium. Rev. Endocr. Metab. Disord. [Internet]. 2025; 26(2):81–96. doi: https://doi.org/pcsp DOI: https://doi.org/10.1007/s11154-024-09923-8
Godbole GB, Modi DN, Puri CP. Regulation of Homeobox a10 expression in the primate endometrium by progesterone and embryonic stimuli. Reprod. [Internet]. 2007; 134(3):513-523. doi: https://doi.org/d2tbh9 DOI: https://doi.org/10.1530/REP-07-0234
Scotti M, Kmita M. Recruitment of 5' Hoxa genes in the allantois is essential for proper extra-embryonic function in placental mammals. Devel. [Internet]. 2012; 139(4):731-739. doi: https://doi.org/fzk7ff DOI: https://doi.org/10.1242/dev.075408
Blitek A, Kiewisz J, Waclawik A, Kaczmarek MM, Ziecik AJ. Effect of steroids on HOXA10 mRNA and protein expression and prostaglandin production in the porcine endometrium. J. Reprod. Dev. [Internet]. 2010; 56(6):643-648 doi: https://doi.org/bzk395 DOI: https://doi.org/10.1262/jrd.10-046K
Topaloglu U, Akbalik ME, Sagsöz H. Immunolocalization of some HOX proteins in immature and mature feline testes. Anat. Histol. Embryol. [Internet]. 2021; 50(4):726735. doi: https://doi.org/pcsq DOI: https://doi.org/10.1111/ahe.12716
Han Y, Su L, Wen YG, Yua FD, Zhua XW, Qiu GQ, Tang HM, Peng ZH, Zhou CZ. Overexpression of HOXA10 promotes gastric cancer cells proliferationand HOXA10+/CD44+is potential prognostic biomarkerfor gastric cancer. Eur. J.Cell Biol. [Internet]. 2015; 94(12):642-652. doi: https://doi.org/f73cpx DOI: https://doi.org/10.1016/j.ejcb.2015.08.004
Sentani K, Oue N, Naito Y, Sakamoto N, Anami K, Zarni-Oo H, Uraoka N, Aoyagi K, Sasaki H, Yasui W. Upregulation of HOXA10 in gastric cancer with the intestinal mucin phenotype: reduction during tumor progression and favorable prognosis. J. Carcinog. [Internet]. 2012;33(5):1081-1088. doi: https://doi.org/f3w6qh DOI: https://doi.org/10.1093/carcin/bgs121
Fiegl H, Windbichler G, Mueller-Holzner E, Goebel G, Lechner M, Jacobs IJ, Widschwendter M. HOXA11 DNA methylation–a novel prognostic biomarker in ovarian cancer. Int. J. Cancer. [Internet]. 2008; 123(3):725-729. doi: https://doi.org/dvsctd DOI: https://doi.org/10.1002/ijc.23563
Hwang JA, Lee BB, Kim Y, Park SE, Heo K, Hong SH, Kim YH, Han J, Shim YM, Lee YS, Kim DK. HOXA11 hypermethylation is associated with progression of non-small cell lung cancer. Oncotarget. [Internet]. 2013; 4(12):2317-2325. doi: https://doi.org/pcsr DOI: https://doi.org/10.18632/oncotarget.1464
Sun Y, Zeng C, Gan S, Li H, Cheng Y, Chen D, Li R, Zhu W. LncRNA HOTTIP-mediated HOXA11 expression promotes cell growth, migration and inhibits cell apoptosis in breast cancer. Int. J. Mol. Sci. [Internet]. 2018; 19(2):472. doi: https://doi.org/gc83nw DOI: https://doi.org/10.3390/ijms19020472
Li Y, Jiang A. ST8SIA6-AS1 promotes hepatocellular carcinoma by absorbing miR-5195-3p to regulate HOXB6. Cancer Biol. Therapy. [Internet]. 2020; 21(7):647-655. doi: https://doi.org/gjvg65 DOI: https://doi.org/10.1080/15384047.2020.1743150
Ignatavicius P, Dauksa A, Zilinskas J, Kazokaite M, Riauka R, Barauskas G. DNA methylation of HOXA11 gene as prognostic molecular marker in human gastric adenocarcinoma. Diagnostics. [Internet]. 2022; 12(7):1686. doi: https://doi.org/g5mvf9 DOI: https://doi.org/10.3390/diagnostics12071686
Hussain I, Bhan A, Ansari KI, Deb P, Bobzean SAM, Perrotti LI, Mandal SS. Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer. Biochim. Biophys. Acta. [Internet]. 2015; 1849(6):697-708. doi: https://doi.org/f7d4q6 DOI: https://doi.org/10.1016/j.bbagrm.2015.02.003
Jung J, Jeong S, Jeong H, Oh HE, Choi JW, Lee ES, Kim YS, Kwak Y, Kim WH, Lee JH. Increased HOXC6 mRNA expression is a novel biomarker of gastric cancer. PLoS ONE. [Internet]. 2020; 15(8):e0236811. doi: https://doi.org/pcss DOI: https://doi.org/10.1371/journal.pone.0236811
Brotto DB, Díogenes-Siena AD, De-Barros II, Silva- Carvalho SC, Muys BR, Goedert L, Cardoso C, Placa JR, Ramão A, Squire JA, Araujo LF, da-Silva WA. Contributions of HOX genes to cancer hall marks: Enrichment pathway analysis and review. Tumour Biol. [Internet]. 2020; 42(5):1010428320918050. doi: https://doi.org/gjtq89 DOI: https://doi.org/10.1177/1010428320918050
Yan Y, Wang R, Hu X, Wang S, Zhang L, Hou C, Zhang L. Mir-126 Regulates properties of SOX9 liver progenitor cells during liver repair by targeting Hoxb6. Stem Cell Rep. [Internet]. 2020; 15(3):706-720. doi: https://doi.org/pcst DOI: https://doi.org/10.1016/j.stemcr.2020.07.005
Yahagi N, Kosaki R, Ito T, Mitsuhashi T, Shimada H, Tomita M, Takahashi T, Kosaki K. Position-specific expression of Hox genes along the gastrointestinal tract. Congenit. Anom. [Internet]. 2004; 44(1):18-26. doi: https://doi.org/b5cdmx DOI: https://doi.org/10.1111/j.1741-4520.2003.00004.x
di Pietro M, Lao-Sirieix P, Boyle S, Cassidy A, Castillo D, Saadi A, Ragnhild E, Fitzgerald RC. Evidence for a functional role of epigenetically regulated midcluster HOXB genes in the development of barrett esophagus. Proc. Natl. Acad. Sci. USA. [Internet]. 2012; 109(23):9077- 9082. doi: https://doi.org/f3zzbt DOI: https://doi.org/10.1073/pnas.1116933109
Li X, Chen S, Zhu Y, Fei J, Song L, Sun G, Niu W, Guo L, Wang J. Comprehensive bioinformatics analyses identified Homeobox B9 as a potential prognostic biomarker and therapeutic target for gastric cancer. J. Gastrointest. Oncol. [Internet]. 2021; 12(5):2132-2149. doi: https://doi.org/pcsv DOI: https://doi.org/10.21037/jgo-21-598
Sakiyama J, Yokouchi Y, Kuroiwa A. HoxA and HoxB cluster genes subdivide the digestive tract into morphological domains during chick development. Mech. Dev. [Internet]. 2001; 101(1-2):233-236. doi: https://doi.org/dwskn2 DOI: https://doi.org/10.1016/S0925-4773(00)00564-5